Over the recent years, research into systemic lupus erythematosus (SLE) has focused on understanding the needs of patients with lupus nephritis (LN), a serious kidney disease caused by SLE. Physicians are interested in incorporating improved diagnosis techniques that can accurately predict the development of LN in SLE patients early on, to employ effective treatments for the control of the disease burden in patients. A recent study has reported the presence of unique RNA segments in the blood serum of patients with SLE, known as microRNAs (miRNAs), which are important regulators of gene expression, and are suggested to be potential predictors of SLE. This discovery has direct implications for the diagnosis landscape of SLE, addressing unmet needs that have been previously identified in the field.
SLE is a long-term autoimmune disease that is characterised by immune cells attacking the body’s own tissues; LN is a manifestation of SLE that targets the kidney, present in 60% of SLE patients (Rojas-River et al., 2023; Yang et al., 2023). As current diagnostic methods include proteinuria quantification in conjunction with kidney biopsy, which are time-consuming and challenging to conduct, there is a need for more efficient diagnosis techniques. In addition, there is a need for early LN diagnosis as patients frequently develop chronic kidney disease, which may lead to end-stage kidney disease, underlining the poor quality of life of patients as the disease progresses (Dörner & Furie, 2019).
Interestingly, the study by Yang and colleagues (2023) compared the levels of exosomal (present outside of cells) miRNA between SLE patients with and without LN. They detected two unique miRNAs (hsa-miR-4796-5p and hsa-miR-7974) at higher levels in SLE patients with LN than in SLE patients without LN, a 1.5-fold difference for both miRNAs, suggesting a predictive role in the diagnosis of LN in patients with SLE. The study also identified these miRNAs’ involvement in signalling pathways that are implicated in the pathogenesis of LN, further revealing a role in LN.
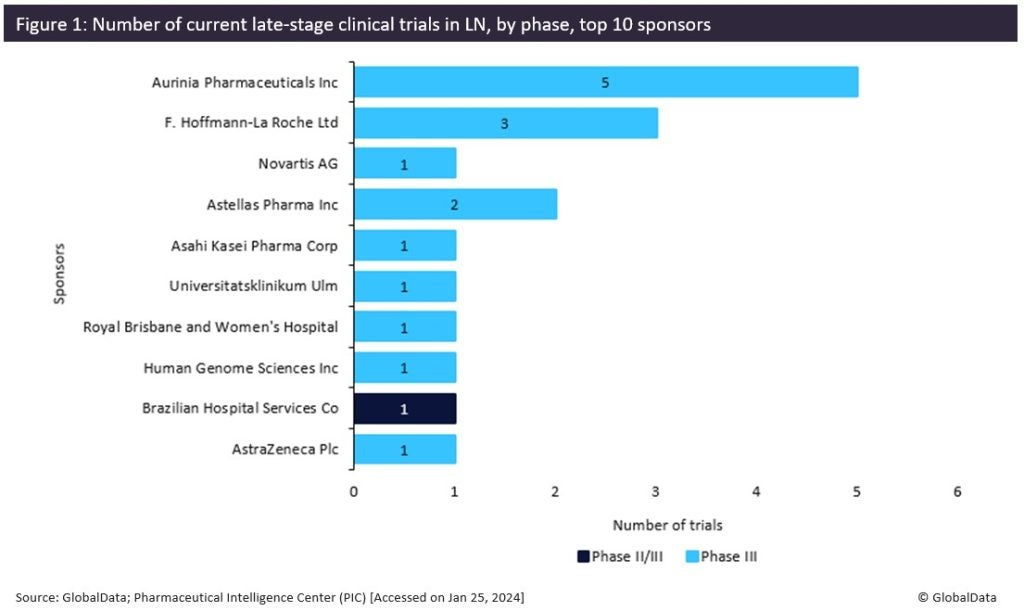
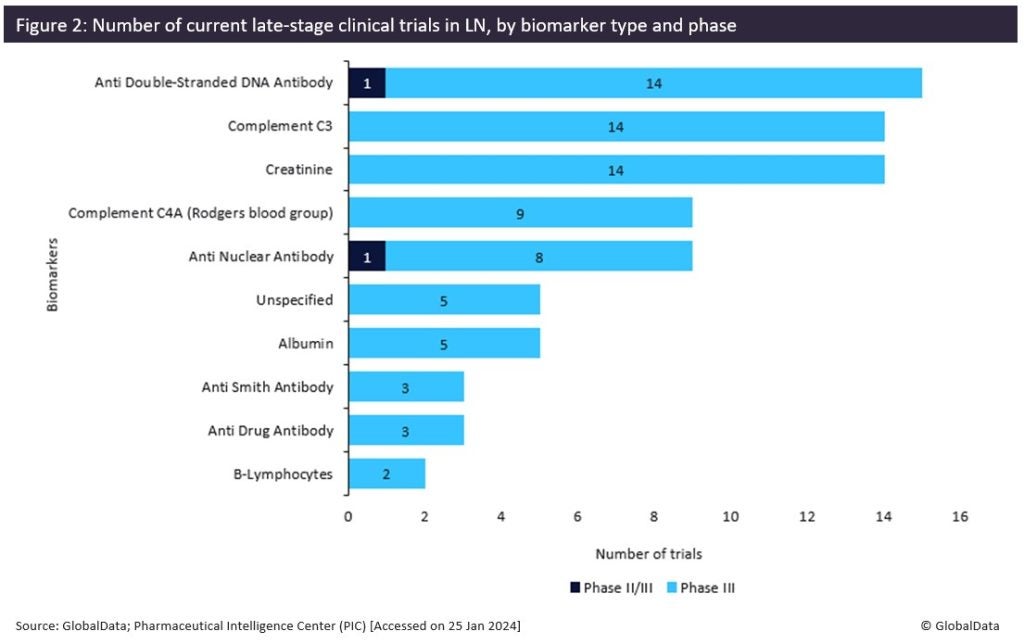
The identification of potent predictors for LN in SLE patients can address unmet needs in the early diagnosis of LN and consequently in the treatment landscape of LN (Piga & Arnaud, 2021). According to GlobalData’s Pharmaceutical Intelligence Center (PIC), multiple drugs have been approved for their use against LN, including Roche’s Rituxan (rituximab) (Japan), Astellas’ Prograf (tacrolimus) (Japan), Aurinia Pharmaceuticals’ Lupkynis (voclosporin) (EU, US), and GlaxoSmithKline’s Benlysta (belimumab) (EU, US), with glucocorticoids and immunosuppressants being the primary therapies for the initial stages of disease progression (Bankole & Nwaonu, 2022). In addition, GlobalData’s PIC has identified the top tencompany sponsors (Figure 1) involved in the late-stage (Phase II/III and Phase III) trials for LN therapies globally, with Aurinia Pharmaceuticals, Novartis, and Roche taking the top three spots of sponsors that have completed or are currently conducting late-stage clinical trials for agents against LN (Figure 1). Furthermore, GlobalData has identified the top ten biomarker types that are targeted in these trials, including anti-double stranded DNA antibodies, the complement component 3, and anti-nuclear antibodies, which are found at the top of the list (Figure 2). Overall, it is apparent that the identification of new predictive molecules for LN in SLE patients will allow the development of advanced therapies in LN which are urgently needed. As demonstrated by GlobalData’s analysis, the competition in the market is high, which further highlights the market opportunity in the field of LN diagnosis and treatment.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData