At the 10th Annual Outsourcing in Clinical Trials (OCT) UK And Ireland conference between 5-6 September 2023, a major theme echoed by experts in the field was the perpetual issue of low accrual and retention in clinical trials.
Lord James O’Shaughnessy revealed that research is not systematically prioritised within the UK’s National Health Service; however, history has shown that increasing clinical staff is not synonymous with reducing disease prevalence within the UK.
Therefore, research into innovative therapies needs to be prioritised to provide the healthcare service with the necessary tools to combat patients’ unmet needs.
He went on to share that the UK has fallen down the global rankings for late-stage clinical research, dropping from fourth to 10th place in Phase III trials between 2017 and 2021.
The lack of success in clinical trials within the UK is perpetuated by the ongoing disparity in the distance between disease-prevalent populations and the clinical trial location.
This sentiment was agreed with by Ian Bruce, director of NIHR Manchester Biomedical Research Centre at the OCT conference, where he pointed out the discrepancy between study sites in proximity to disease prevalence throughout Manchester.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
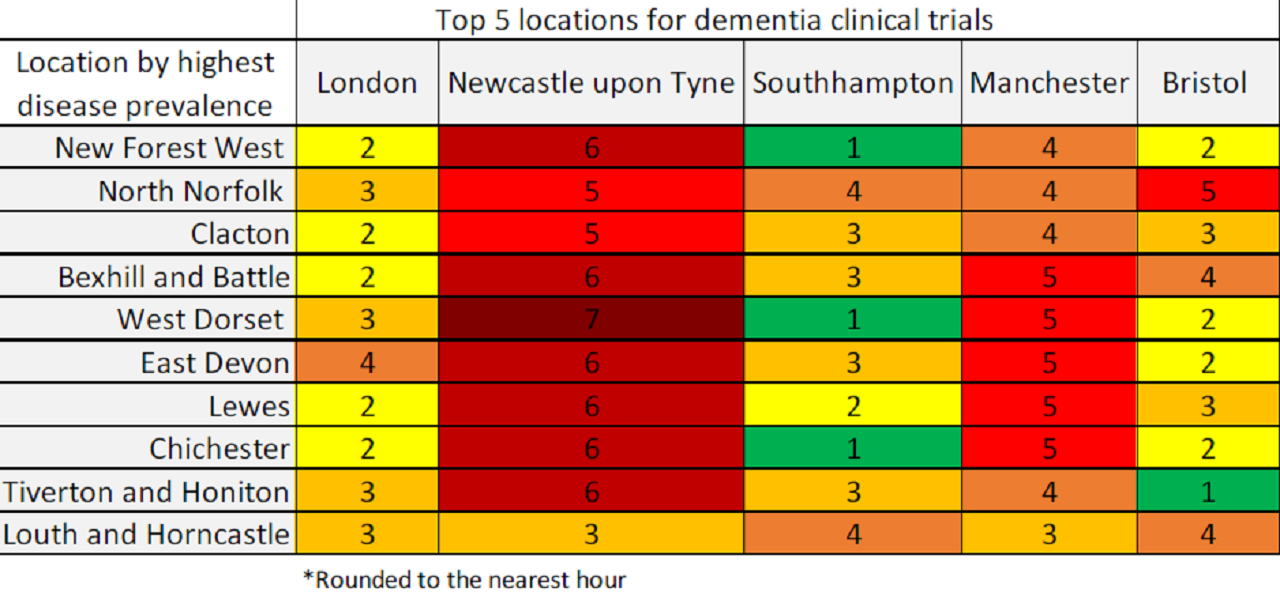
By GlobalDataUsing a combination of Alzheimer’s research, UK dementia prevalence by parliamentary constituency, and GlobalData’s clinical trials database, the travel distance between the top five locations in England for dementia clinical trials, and the locations with the highest dementia prevalence was calculated (Table 1).
This revealed that 72% of the top ten dementia-prevalent locations are at least a three-hour drive away from the leading locations for clinical research in the field, and only 8% of the top ten dementia-prevalent locations are within a one-hour drive to the leading locations for clinical research.
This means that a staggering number of potential participants living with dementia are not gaining access to promising therapies in trials and subsequently thereafter, as often trials are unsuccessful due to low accrual and retention.
This perpetual cycle permeates countless therapy areas throughout the UK and beyond.
Several practical suggestions were provided throughout the OCT conference to prevent patients’ proximity to a site from impacting their participation in a clinical trial, as well as increasing a trial’s geographical reach.
One suggestion was the use of decentralised clinical trials (DCTs), as these completely remote trials provide flexibility for participants, increase recruitment of a more diverse patient pool, and aid retention.
However, DCTs are only suitable for well-characterised drugs with minimal safety concerns, meaning they are inappropriate for most clinical trials.
This was echoed at the conference by Sverre Bengtsson, co-founder and senior vice-president of Strategic Relations at Videdoc, who revealed companies are pulling away from the DCT approach as it currently stands.
He then went on to acknowledge the value of hybrid trials that utilise DCT components; these trial designs generally have increased flexibility and are applicable to a wider diversity of clinical trials, so can be a great compromise between a traditional trial and a decentralised trial.
There is no single solution to fix the ongoing issues surrounding patient accrual and retention.
However, a spotlight on clinical sites’ proximity to the actual disease-prevalent populations is long overdue.
Getting this balance right has the potential to impact the underperformance of clinical trials within the UK and therefore restore its global ranking, and most importantly increase patient access to industry clinical trials.