Electronic consent (e-consent) is software that allows trial participants to read and sign informed consent documents online.
The use of e-consent increased rapidly during the Covid-19 pandemic as clinician research staff were unable to deliver paper forms to participants.
While e-consent was mostly considered to be a means to an end during the pandemic, it has since been recognised as an accessible, effective, and patient-centric solution.
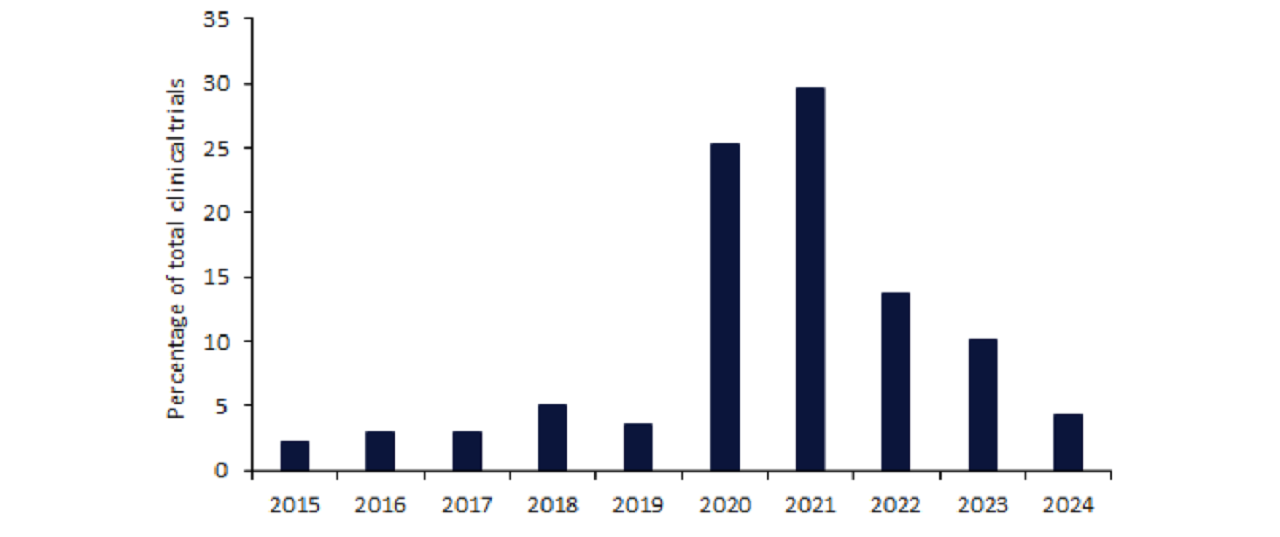
According to leading data and analytics company GlobalData’s Trials Intelligence platform, the number of clinical trials utilising e-consent in 2020 was 600%
higher than in 2019.
The continued disruption caused by the Covid-19 pandemic saw this figure increase by an additional 17% in 2021.
As this software became more widely adopted, more of its advantages were recognised beyond its initial purpose of bypassing restrictions imposed during the pandemic such as the way that it can improve a participant’s experience.
E-consent provides participants with more time to carefully review and understand documents within the familiar comfort of their homes and can include comprehension measures to determine the extent that the information is being
understood.
A study performed by the Center for Info and Study on Clinical Research Participation revealed that around 61% of participants find e-consent forms to be very easy to understand, compared to 41% of participants when asked the same question about paper consent forms.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAlthough this reveals that more must be done to improve comprehension across both digital and paper consent forms, e-consent already appears to be drastically in the lead.
One way to improve the quality of e-consent is to meet participants where they are such as by prioritising a participant-friendly intuitive interface so that people with all levels of technical abilities are considered.
This was a sentiment echoed by Christine Von Raesfeld, founder and CEO of People with Empathy, at the Outsourcing in Clinical Trials West Coast 2024 conference held on 6-7 February 2024.
Regarding information shared with participants throughout a trial, she said: “It’s
not about dumbing things down to a sixth-grade level, it’s about bringing the information to them in the language they speak”.
Providing participants with this patient-centric option will ultimately increase trial success.
Data shared by WCG revealed that 35% of participants who dropped out of a clinical trial thought the informed consent document was hard to understand.
As such, prioritising patients’ comprehension will increase retention and enrollment rates, as well as reducing paperwork and the associated administrative duties.
Despite this, GlobalData’s Trials Intelligence Platform shows that the
post-pandemic utilisation of e-consent dropped by more than 50% in 2022, and the decline continued into 2023.
However, 2024 appears to be off to a strong start, as within the first two months of the year, there have already been almost half the number of trials using e-consent that were reported in all of 2023.
Hopefully, e-consent will be given the opportunity to positively impact the clinical trials landscape.