Human immunodeficiency virus (HIV) is a virus that attacks the immune system – cells that help the body fight infection. It can spread through contact with blood, semen, or vaginal fluids. If untreated, HIV will then advance into acquired immunodeficiency syndrome (AIDS). As symptoms may take years to develop, many people living with HIV currently do not know they have it. Therefore, it is important to get regularly tested. National HIV Testing Day is observed in the US on 27 June.
There are many benefits to getting tested regularly. It is the first step in preventing transmission and also helps maintain a healthier life. Currently, there is no cure for the virus, but many medications help control the infection. Clinical trial sponsors continue to test various candidates to find a treatment.
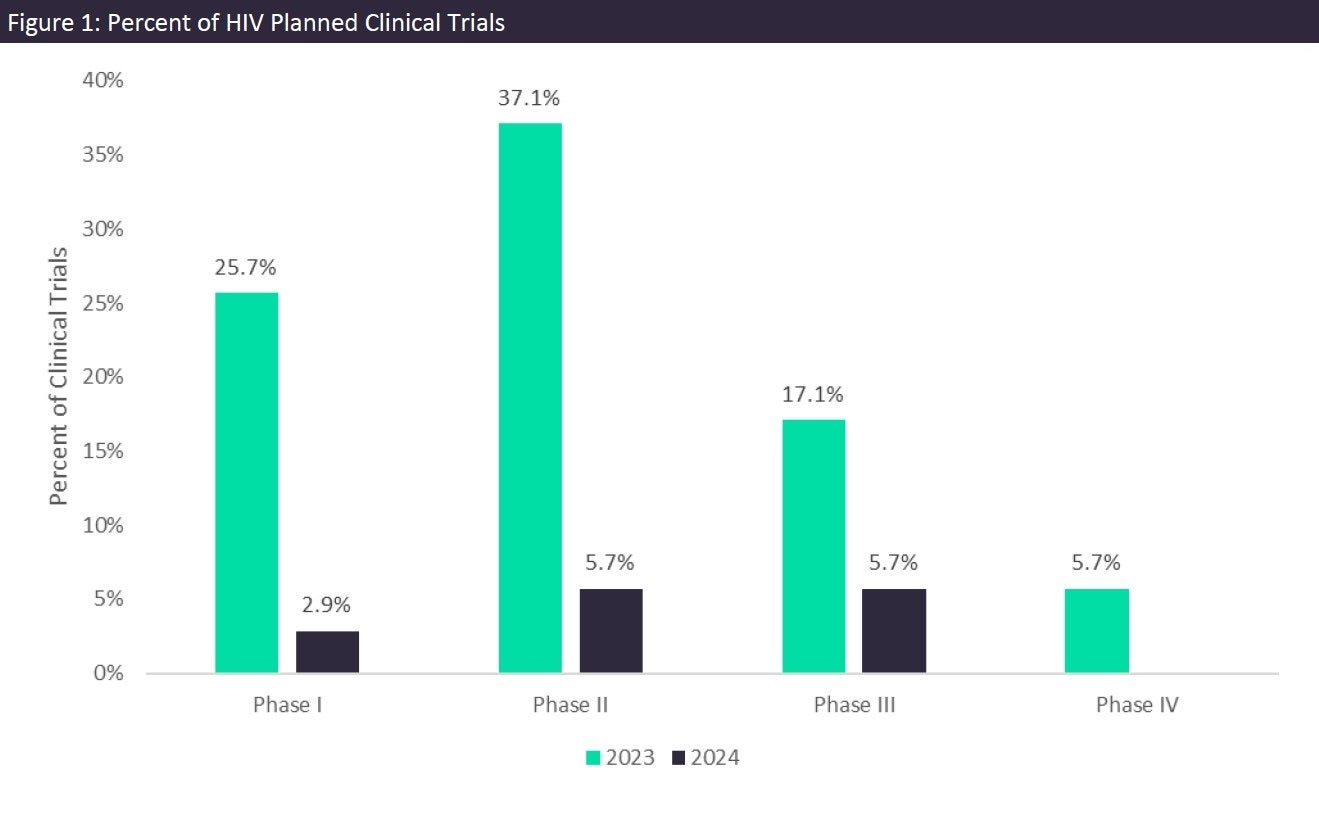
GlobalData’s Clinical Trials Database tracks all HIV/AIDS clinical trials. Currently, there are over 7,000 HIV clinical trials in all phases and statuses, including numerous planned trials to begin in 2023 and 2024. When examining these planned trials, the majority of them are expected to begin in 2023, with the most in Phase II (37.1%) followed by Phase I and Phase III trials, as seen in Figure 1. The top sponsors, Gilead Sciences and United BioPharma, are going to test their lead drug candidates in six total trials.
Gilead Sciences will initiate two Phase I trials on 26 July 2023 and one Phase II trial on 1 November 2023 using lenacapavir sodium LA, also known as Sunlenca. Lenacapavir sodium is a first-in-class, long-acting HIV capsid inhibitor. It is formulated as long-acting film-coated tablets for oral administration and as a solution for subcutaneous injection. The Phase I trials will be enrolling 50 adult patients each while the Phase II trial will enrol children who are under the age of
18.
United BioPharma plans to initiate its three trials, one in each phase (Phase I, Phase II, and Phase III), in December 2023. All trials will be testing semzuolimab, or UB-421. UB-421 works by binding to the CD4 protein on the cell surface, where it blocks or displaces HIV viruses and blocks entry by all major primary strains of HIV into susceptible human host cells. The neutralising activity of UB-421 blocks HIV-1 from binding to its receptor on CD4-positive cells. Thus, UB-421 functions as an immunotherapeutic intervention to prevent HIV-1 infection.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData