On 9 October 2023, AnapstysBio made the results of a randomised Phase III clinical trial public, which included the administration of a novel inhibitor, imsidolimab, to patients with generalised pustular psoriasis (GPP) flares, demonstrating promising outcomes for the improvement of GPP flares.
As the drug candidate is an anti-interleukin-36 receptor (IL-36R) antibody and acts as an antagonist of IL-36R signalling, the latest set of results strengthens imsidolimab’s case as a potential treatment for patients with pustular psoriasis.
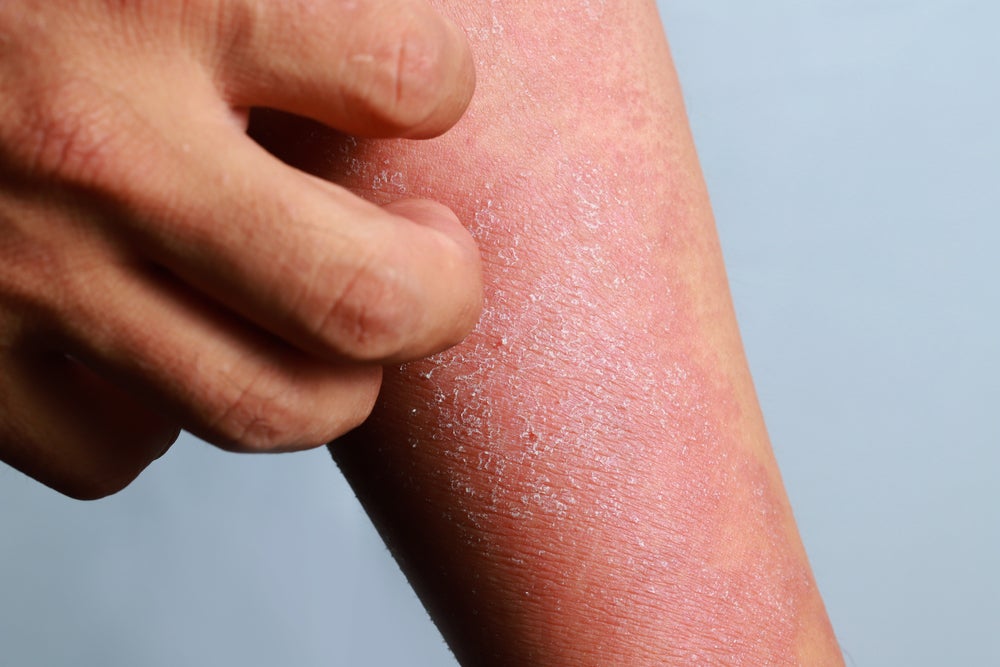
GPP is a rare subtype of psoriasis and patients exhibit painful sterile pustules complemented with a burning feeling in the skin. The incidence of GPP is correlated with mutations in the IL-36R signalling pathway and the upregulation of IL-36 function leads to uncontrolled inflammatory episodes and incidence of pustular psoriasis.
The administration of imsidolimab in the study demonstrated the rapid clearance of GPP, which was observed in 53.3% of the participants that had been administered with 750mg intravenous imsidolimab at week four, meeting the primary endpoint.
The exciting news for the GPP treatment landscape comes after a recent publication that highlighted the unmet need for the treatment of flares in patients with GPP.
Specifically, the publication (Zema et al, 2022) identified that out of the 1,535 participants who were patients with GPP, 271 patients exhibited 513 flares, and demonstrated a high comorbidity burden, resulting in more frequent emergency hospital visits and high use of all treatment classes.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataInterestingly, the lack of use of advanced treatments upon flare-up incidences was identified, suggesting an unmet need in the treatment of flare-ups in patients with GPP.
AnaptysBio is a clinical-stage biotechnology company that focuses on the development of antibody candidates for the treatment of multiple diseases including atopic dermatitis, multiple types of cancer and autoimmune indications, as well as asthma and psoriasis.
The San Diego, US-based company utilises a proprietary antibody discovery technology platform using somatic hypermutation and currently possesses multiple product candidates under development for the treatment of diseases such as moderate-to-severe adult atopic dermatitis, asthma, and GPP.
The company also has partnerships with GSK to develop a number of product candidates for therapy areas such as immuno-oncology.
According to GlobalData, Boehringer Ingelheim’s Spevigo (spesolimab) is the only treatment that has been approved by the US Food and Drug Administration (FDA) specifically to treat flares of GPP, and is currently considered to be imsidolimab’s main competitor should the latter receive approval for GPP in the US (EMA, 2023).
Spevigo (spesolimab) has also been approved in other markets such as South Korea, Switzerland, Canada, France, Spain, Germany, Japan, Brazil, Italy, Austria, the UK, Denmark, and China, according to GlobalData.
Other drugs such as AbbVie’s Skyrizi (risankizumab) (AbbVie, 2019), Novartis’s Cosentyx (secukinumab) (Novartis, 2022), UCB’s Cimzia (certolizumab pegol) (UCB, 2022a) and Bimzelx (bimekizumab) (UCB, 2022b), Kyowa Kirin’s Lumicef (brodalumab) (Kyowa Kirin, 2021) and Bristol Myers Squibb’s Sotyktu (deucravacitinib) (Hoy, 2022) have all been approved in Japan for GPP, however, their primary use is not for the treatment of GPP.
Nevertheless, it is suggested that further clinical results may provide a thorough understanding of the future of imsidolimab in the field of psoriasis and specifically GPP.