As the Covid-19 pandemic becomes endemic, it continues to have lasting effects, especially with lingering symptoms in those who were diagnosed with the infectious disease. The National Institute of Health (NIH) named these lasting symptoms post-acute sequelae of SARS-CoV-2 infection (PASC), also known as long Covid. Long Covid consists of a wide range of conditions that can last for weeks, months, or even years after infection. The most common symptoms include tiredness and fatigue, shortness of breath, and change in smell or taste. Certain people or groups are at a higher risk of developing these conditions, including individuals who experienced a more severe Covid-19 illness, those who had underlying health conditions prior to having Covid-19, and those who did not get a Covid-19 vaccine.
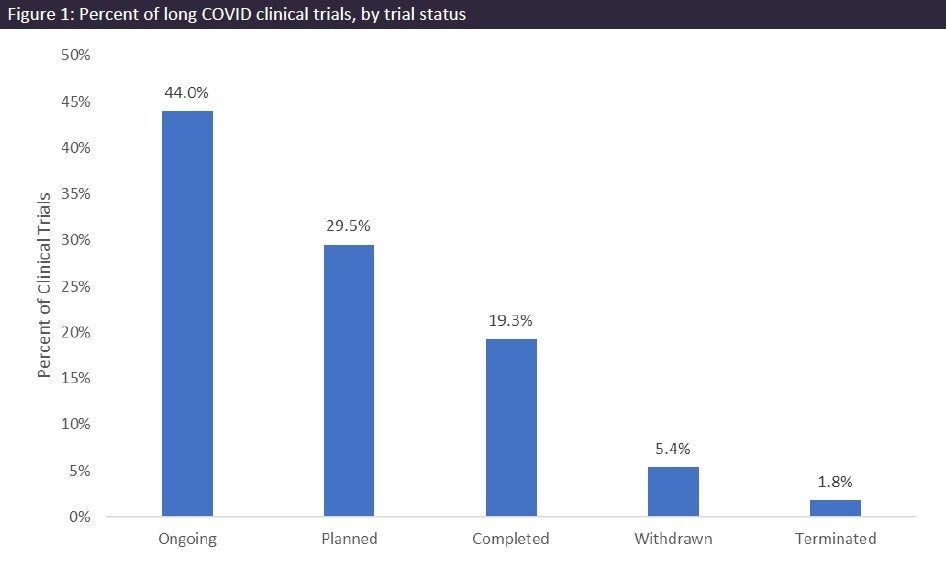
According to GlobalData’s Clinical Trials Database, there are more than 150 long Covid clinical trials, with the most being currently ongoing (44%), as seen in Figure 1 (above). Planned trials follows with 29.5%, and then completed is the third most common status with 19.3%. The top drug candidate being tested for the treatment of long Covid is colchicine. Colchicine is an anti-inflammatory drug used to treat a wide variety of conditions, such as gout and pericarditis. It is currently marketed in the US, the UK, Spain, France, South Africa, Switzerland, and Austria for the treatment of gout.
According to GlobalData’s Catalyst Calendar, there are two trial events happening for colchicine for the treatment of long Covid, with both being Phase III trial completions. First, The George Institute for Global Health expects to complete its 350-patient trial, ‘Colchicine to reduce Covid-19 related complications in high-risk patients post-Covid-19 infection’, in November 2023. It is testing colchicine as a monotherapy. Second, The University College London’s 4,520-patient trial, ‘Symptoms, trajectory, inequalities and management: understanding long Covid to address and transform existing integrated care pathways’, is expected to be completed in May 2024. This trial is using colchicine and other drugs such as famotidine, rivaroxaban, and loratadine as combination therapies.