GlobalData analysed the number of clinical trials examining cystic fibrosis with start dates between January 1, 2009 and August 28, 2019.
Cystic fibrosis is a hereditary genetic condition that is caused by the occurrence of recessive genes for the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Two carriers of the recessive allele can produce offspring that have a mutation for CFTR. The function of the CFTR protein in humans is to regulate mucus production, sweat, and digestive fluids. If a genetic mutation occurs, CFTR can become dysfunctional, causing thick mucus secretions and extreme difficulty in breathing.
Screening programs for newborns occur in certain countries, but the condition can also be diagnosed by genetic testing and pancreatic observations. There is no current cure or prevention for cystic fibrosis. However, gene therapy trials researching the genetic mutations that cause CFTR dysfunction are currently underway. Treatments help manage the disease and increase patient life expectancy, which currently stands at 42–50 years.

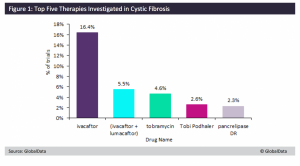
GlobalData has determined that the main therapy drug for the management of cystic fibrosis, by count of clinical trials, is Ivacaftor (KALYDECO®, Vertex Pharmaceuticals) (16.4% of trials), as shown in Figure 1. Ivacaftor is the first drug that treats the underlying cause of cystic fibrosis rather than solely focusing on symptoms. The drug works by improving the movement of chloride through ion channels by keeping the channels from constricting. This helps decrease the accumulation of thick mucus and helps maintain a balance of salt and water in the lungs. Similarly, a combination therapy of Ivacaftor + lumacaftor (ORKAMBI®, Vertex Pharmaceuticals) (5.5% of trials) is also used to treat underlying issues caused by cystic fibrosis. Lumacaftor works by increasing the number of CFTR proteins that are passed onto the cell surface while Ivacaftor keeps the ion channels open.


US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe majority of cystic fibrosis trials analyzed by GlobalData were in Phase II (40.6%), followed by Phase I (25.9%). Most of these trials were led by non-industry sponsors (46.5%). Among the top five countries to conduct Cystic Fibrosis trials, the US had the highest amount at 73.6%.