Historically, female participants have been underrepresented in clinical trials as medical research has been more focused on male health. Studies have shown that this lack of female inclusion correlates to suboptimal health care and adverse medical outcomes for women. Closing the gender health gap has become a topic of paramount concern in recent years. Adequate female trial participation is crucial to analyze sex-specific differences in data to prevent women from experiencing poorer health outcomes than men. However, although GlobalData’s clinical trials database has revealed that there are several conditions that disproportionately affect women, the participant demographic is exceedingly male.
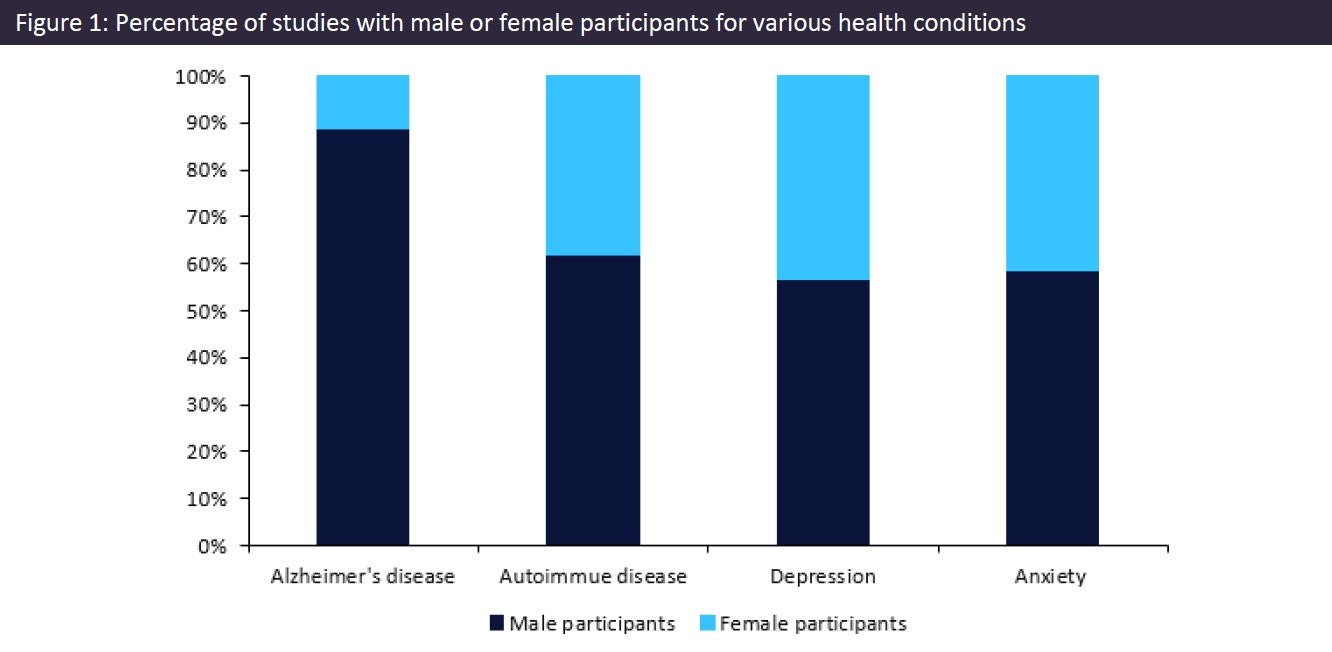
Figure 1 (above) presents the discrepancy between clinical trials that included male or female participants for Alzheimer’s disease (AD), depression, anxiety, and autoimmune diseases, which are all more prevalent in women. According to Alzheimer’s Society, around twice as many women have AD than men. However, clinical trials in AD include eight times more male participants than female participants. Similarly, the World Health Organization (WHO) confirms that women are twice as likely to have depression or anxiety, but most studies in these indications use male participants. Additionally, while the National Institute of Health confirms that 80% of individuals with an autoimmune disease are women, the clinical trials in this indication use twice as many male participants as female participants.
GlobalData’s Clinical Trials database also indicates that women are underrepresented in Phase I clinical trials, which are vital for determining how a drug interacts with the human body, as well as the safety, optimum dose, and side effects of the drug, which may differ depending on the sex of the participant. This data reinforces that the underrepresentation of female participants in clinical trials is not a thing of the past, but a perpetual issue.