
Gene therapy is a technique involving the delivery of genetic material via a vector into a patient’s cells to remedy the effects of a defective gene. Over the last two decades, the field of gene therapy has evolved significantly due to innovations in research concerning neuro-degenerative disorders, genetic diseases and numerous cancers. Diseases previously regarded as incurable may now have viable treatment options and permanent cures. In this analysis, we examine the evolution of trials in the field of gene therapy and analyse how the geographic distribution of these studies has transformed.
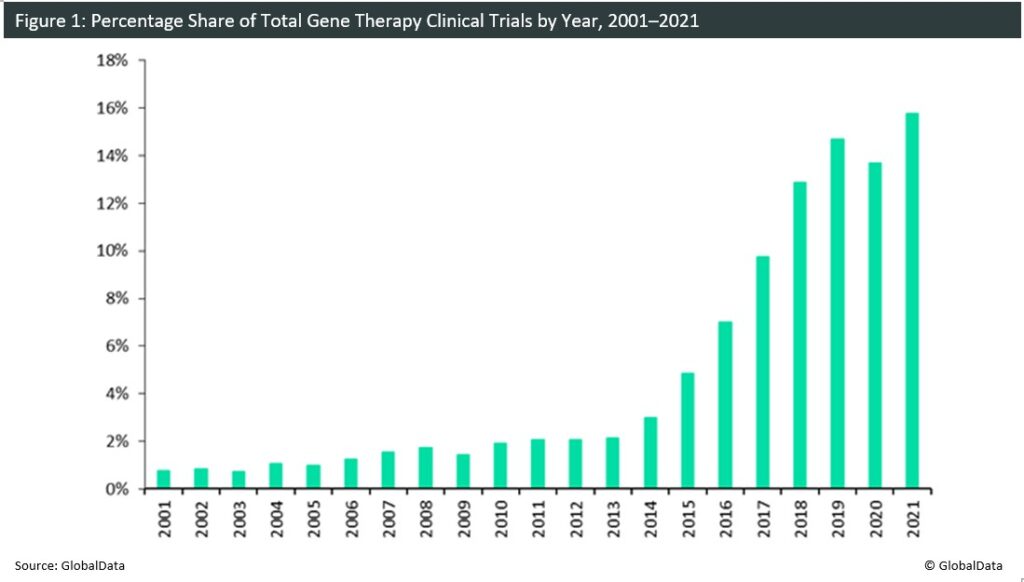
There has been an increasing number of newly initiated gene therapy clinical trials over the last 20 years. In the early 2000s, the field was in an uncertain position. Trials conducted during this period were impacted by safety concerns, including the first patient death in 1999 following gene therapy and the development of leukaemia linked to the use of adenoviral vectors in 2002. In 2003, China was the first country to approve the commercial production of the gene therapeutic Gendicine, which is indicated for head and neck cancer. However, the attitudes of regulatory bodies in the US and Europe at this time were considerably more conservative due to the safety concerns highlighted by clinical trials during this period. In 2012, there was a defining shift in gene therapy research following the approval of UniQure’s Glybera (alipogene tiparvovec) by the European Medicines Agency for lipoprotein lipase deficiency, making it the first gene therapy to be approved in Europe. In subsequent years, there was a significant increase in clinical trial initiations, with record increases seen every year from 2013 to 2018. The greatest increase in trial initiations was observed during 2017–2018 when the FDA approved the first gene therapy agent in the US, Novartis’ Kymriah (tisagenlecleucel) acute lymphoblastic leukemia. The approval evidently boosted the confidence of new sponsors in the field. In 2020, the first decrease in trial initiations since 2009 was observed. This decline can be primarily attributed to the disruptive effects of the Covid-19 pandemic on clinical trials. Nonetheless, 2021 has already presented with a record number of trials as sponsors have become better equipped in navigating the pressures of the pandemic.
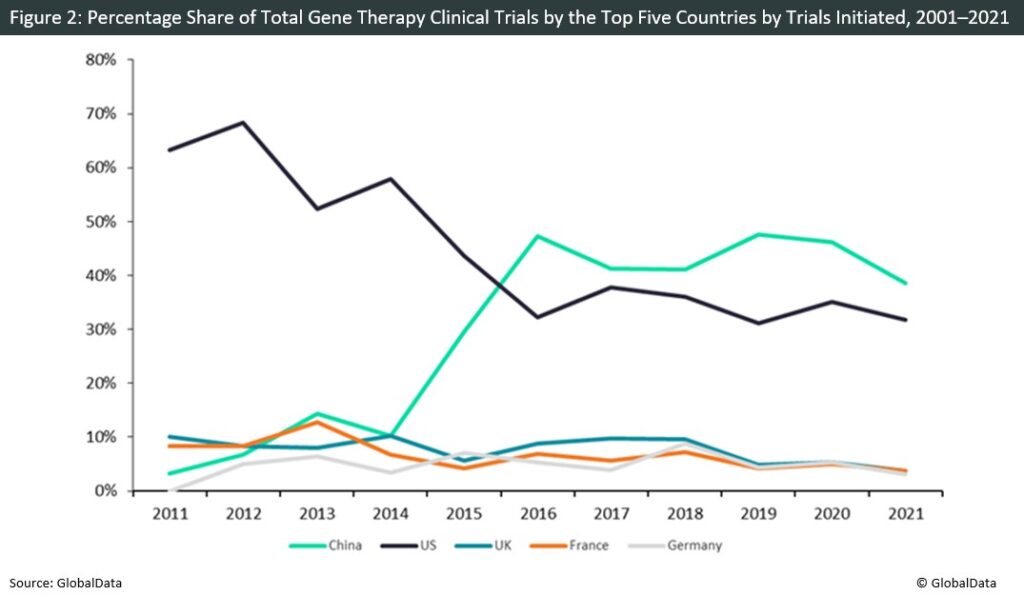
The US assumed research dominance until 2016, where an upsurge in the number of clinical trials in China became evident. Since then, China has become the principal research location for gene therapy trials, leading trial initiations every year from 2016 onward. This rapid growth may be attributed to a variety of factors, primarily China’s expanding research ecosystem and significant governmental support for emerging technologies. In 2016, China’s State Council published the government’s five-year industrial development strategy for emerging technology, which incorporates the acceleration of the pace of innovation and development of the biotech industry, including the development of new gene therapies. This period marks China’s dominance in the field of gene therapy. Furthermore, where research output is concerned, China is the only country in the top five where non-industry sponsored trials outnumber industry-sponsored trials (73% versus 27%). This highlights the fact that China’s gene therapy research is being spearheaded by a significant governmental funding push, especially through institutional research to foster innovation in the field of gene therapy. Urgent demand also exists for new therapeutics in China, with an estimated 57 million people suffering from genetic disorders, more than four times the number of sufferers in the US. Evidently, the large patient population means that China is considered an attractive location for gene therapy clinical studies and commercialization.
However, one potential factor influencing the surge in studies may be potentially less-stringent regulatory requirements in China relative to other regions. During the pandemic, there was a greater drop in the share of studies in China compared to the US. Notably, several ethical and safety concerns were raised by the 2019 gene-editing controversy in which a researcher named He Jiankui carried out gene editing in human embryos using CRISPR technology. This led to increased scrutiny surrounding gene editing and therapy in China. Coupled with the effects of the pandemic, a notable reduction in China’s research efforts is evident. As such, it will be interesting to see whether the US or China will serve as the industry research leader in the next few years.
Following the research boom spearheaded by Glybera’s European approval, European countries have failed to adequately keep up with the level of research observed in the Asia-Pacific region and North America. This may be influenced by the progress of Glybera, as the therapeutic proved to be highly expensive, costing in excess of $1m per patient and with patients typically unable to afford the treatment outside of clinical trials, thus impacting its sales. This led to the sponsor terminating the Phase IV studies that were required to extend the existing EU conditional market approval. Therefore, Glybera failed to demonstrate commercial success in Europe, potentially slowing the growth of gene therapies in the region.
Cell & Gene Therapy Coverage on Clinical Trials Arena supported by Cytiva.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataEditorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.




