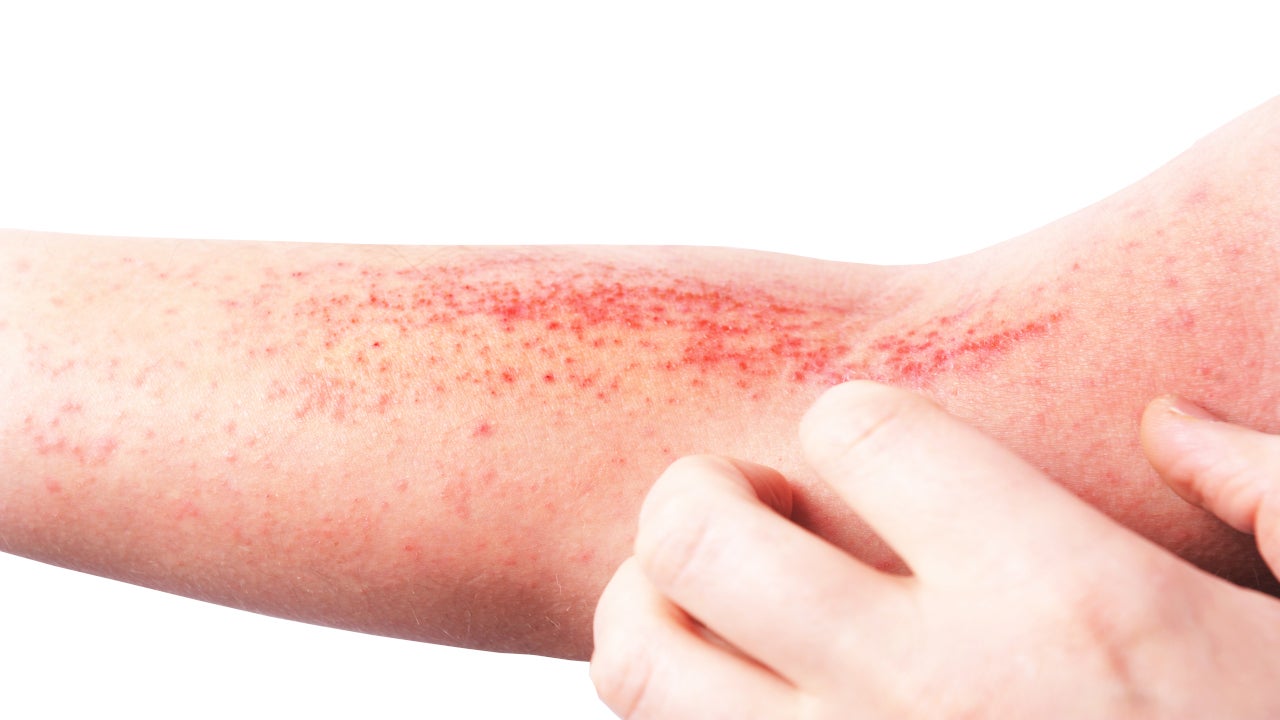
Aclaris Therapeutics’ ATI-1777 Likelihood of Approval (LoA) in moderate-to-severe atopic dermatitis rose by 22 points to 47% after positive topline results were revealed from a Phase IIa trial on 8 June. The asset’s LoA was previously 25%, which was updated on 11 June. The LoA is identified via GlobalData’s analysis using a combination of machine learning and its proprietary algorithm.
In the 50-patient Phase IIa trial, participants were divided into two groups: one receiving topical ATI-1777 JAK 1/3 inhibitor and the other group placed into the vehicle arm. In the primary endpoint modified Eczema Area and Severity Index (mEASI) score at week 4, ATI-1777 demonstrated statistically significant result versus the comparator arm. Final Phase IIa results will be submitted in a peer-reviewed scientific journal for publication, as per the 8 June media release.

Access deeper industry intelligence
Experience unmatched clarity with a single platform that combines unique data, AI, and human expertise.
Aclaris indicated it is planning to advance ATI-1777 to the next stage of development in in moderate-to-severe atopic dermatitis. The topical’s Phase Transition Success Rate (PTSR) also jumped by 23 points to 50%. PTSR is the probability, given as a percentage, of a drug progressing successfully from one development stage to the next. The LoA is calculated by compounding the PTSR at each stage the drug is yet to progress through.
Aclaris has a $1.07bn market cap.
Reynald Castaneda is an Associate Editor for Clinical Trials Arena parent company GlobalData’s investigative journalism team. A version of this article originally appeared on the Insights module of GlobalData’s Pharmaceutical Intelligence Center. To access more articles like this, visit GlobalData.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData