EU Clinical Trial Regulation: A Guide for Sponsors
Keep up to date with our rolling coverage on the Clinical Trial Regulation via visiting our recent stories below the page.
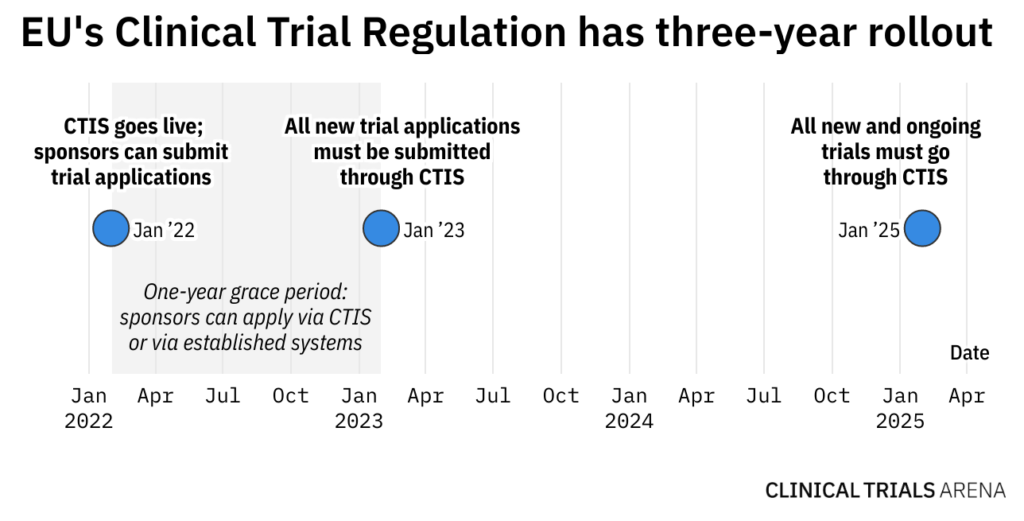
The European Medicines Agency (EMA) has announced that its long-delayed clinical trial EU Portal and Database, one of the main features of the Clinical Trial Regulation 536/2014 and the key component of the Clinical Trial Information System (CTIS), is now finally fully functional and fit for purpose with 31 January 2022 pencilled in as the go-live date.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The functional specifications of the portal and database were drawn up in collaboration between the EMA, European Commission (EC) and EU member states and were confirmed during a meeting held on 21 April following an independent audit of the new system.
The portal is designed to make it easier for trials to be conducted in the EU across multiple member states and to increase transparency around clinical trials for the public.
Although the Clinical Trial Regulation was adopted and entered into force in 2014, its full application has been dependent on this confirmation of full functionality of CTIS, which was originally expected in September 2018 but had been stunted by various delays.
EU trial regulation inches towards a transformation
The CTIS is the final piece of the puzzle that makes up the EU’s ambitious remodel of the clinical trial system on the continent. The Clinical Trial Regulation has been waiting in the wings for this development as it will only apply upon the sign-off of the CTIS, meaning the regulation will apply on the same date that the CTIS goes live.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIf the CTIS operates as it should it would be a huge step forward, considering the discourse around digitising healthcare systems has dominated industry discussions and conferences over the past decade with little movement or success seen on any meaningful scale.
“We are all prepared to start working with the system,” said EMA management board chair Christa Wirthumer-Hoche. “The implementation of the Clinical Trial Regulation and CTIS will increase efficiency in the registration, conduct and supervision of clinical trials in the EU, particularly those taking place in multiple Member States, while ensuring utmost transparency for the public. This is one of the most complex and ambitious IT developments carried out by EMA and we look forward to its go-live in early 2022.”
“The EU is an attractive location for investment in clinical research and this development will further enhance its value as a large and dynamic clinical research area, enabling authorities and researchers to cooperate more effectively across Member States,” added Belgian Federal Agency for Medicines and Health Products CEO Xavier De Cuyper. “It also means we can further enhance the benefits for our citizens of new medicines, and better use of existing medicines, and provide them with access to increased public information on clinical trials as they are ongoing and their results when the trials are completed.”
The Clinical Trial Regulation: boosting efficiency
The way clinical trials are conducted in the EU will undergo a major transformation once the Clinical Trial Regulation comes into effect. The regulation will replace the existing Clinical Trials Directive 2001/20/EC and will harmonise the registration, assessment and supervision processes for clinical trials throughout the EU via the CTIS. In contrast, under the current Clinical Trials Directive, national rules around the assessment and conduct of trials vary from one member state to another.
The regulation’s aim is to create a landscape that is “favourable to conducting clinical trials in the EU, with the highest standards of safety for participants and increased transparency of trial information,” the EMA has said.
The hope is that it will increase the efficiency of all trials in Europe, especially for those conducted across multiple member states, and foster innovation and research in the continent while helping avoid duplication of clinical trials or repetition of those that have been unsuccessful.
CTIS: a central portal for EU trials
CTIS will contain the centralised EU portal and database, and when live, will be the single EU entry point for clinical trial applications. It will enable trial sponsors to apply for a clinical trial in all countries of the European Economic Area (EEA) with a single application rather than having to apply separately in every country. This one-and-done application will include the submission to national competent authorities and to the ethics committees for all involved countries.
The authorisation and oversight of clinical trials will remain the responsibility of member states but the EMA will set up and manage CTIS in collaboration with the member states and the EC.
The centralised system will allow all clinical trial stakeholders including sponsors, researchers and national competent authorities to collaborate and communicate across borders, hopefully leading to better outcomes and knowledge-sharing.
“The system will support the day-to-day business processes of member states and sponsors throughout the life-cycle of a clinical trial in a user-friendly way,” said the EMA. “It will provide regulatory oversight of clinical trials and tools for supervision and monitoring,” and will “contain collaboration tools, workflow and document management capabilities, accessible via individual workspaces”.
The CTIS will also be a boon for patient recruitment to trials by allowing sponsors and researchers to easily expand trials to sites in other EEA countries.
It will house a public website to be supervised by the EMA, similar in intent (if not in scope) to the US National Institutes of Health’s ClinicalTrials.gov database. The European portal will contain detailed information on all clinical trials conducted in the EU and their outcomes throughout their lifecycle, thus improving transparency and access to information for patients, healthcare workers and researchers.
The data available in CTIS excludes personal data, commercially confidential data, commercial communications between member states and information about clinical trial applications that have not yet been approved.
Going live: what happens next?
Once the European Commission confirms the system meets the conditions of the Clinical Trial Regulation it will publish a notice announcing the functionality of the CTIS in the Official Journal of the European Union.
The CTIS will go live six months after the date of publication of the notice, initiating the application of the Clinical Trial Regulation.
According to the EMA: “It is the desire of the Board, EMA and the European Commission that the system goes live on 31 January 2022, which would imply that the Commission notice in the Official Journal would be published on 31 July 2021.”
Read more:
No time to delay: sponsors need to act fast ahead of CTIS deadline
With all new clinical trial applications expected to go through the new system from January 2023, CTIS uptake is still slow. Read more…
Are you ready? EU’s Clinical Trials Regulation and portal goes live 31 January
The EU’s Clinical Trials Regulation and information system aims to streamline applications and bolster transparency – but initial rollout could face growing pains. Read more…