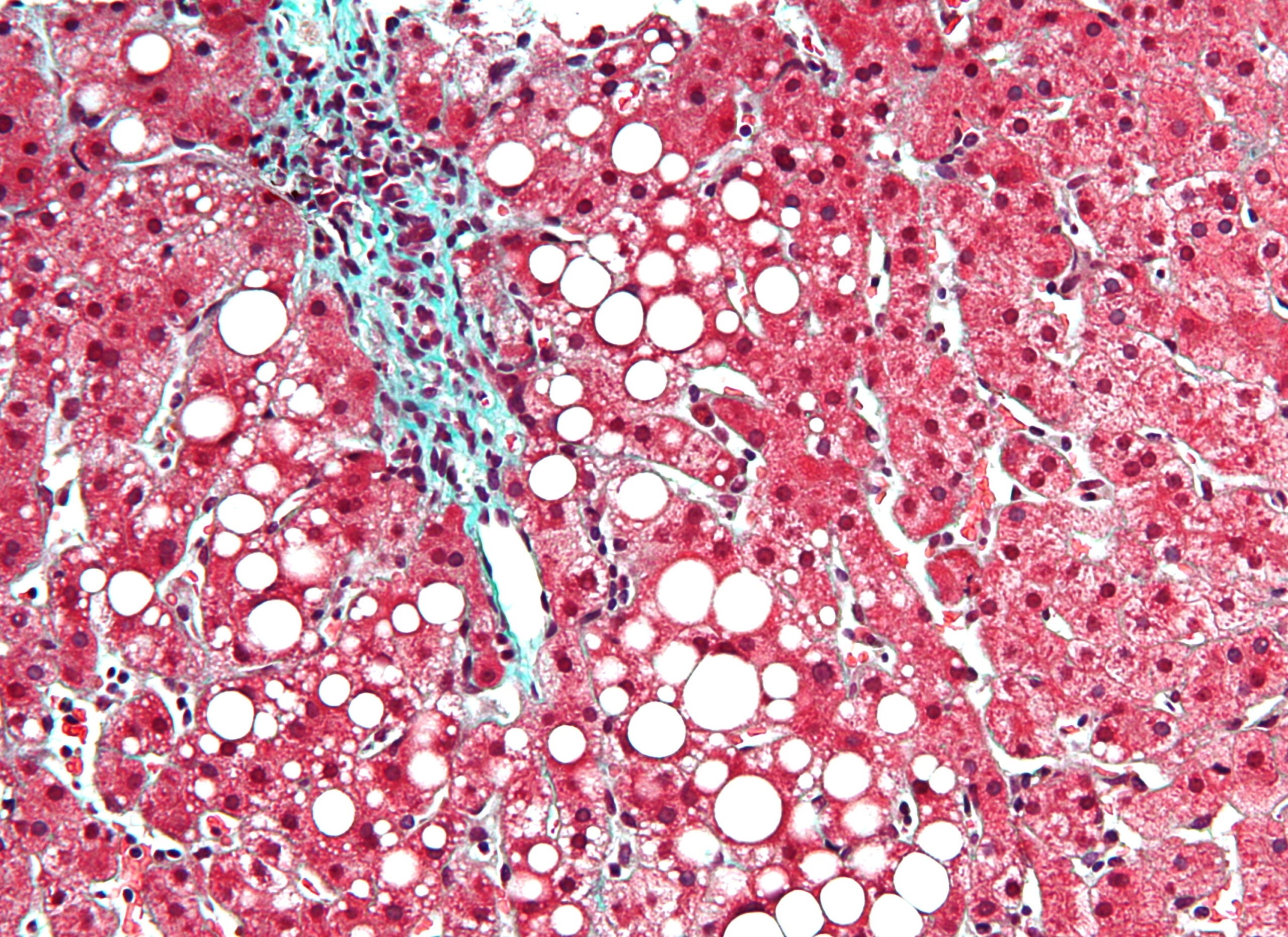
Sagimet Biosciences is using digital pathology in its Phase IIb nonalcoholic steatohepatitis (NASH) trial in a bid to attain more consistent biopsy analysis, CEO George Kemble says. In NASH, digital pathology involves histological imaging of biopsy samples, which are then analysed by artificial intelligence (AI) to detect fibrosis changes.
In NASH clinical trials, classifying patients between the different fibrosis stages can be tricky because of pathologist bias. To accommodate for inconsistent biopsy readings between pathologists, some trials have three different experts that review samples, with the third pathologist breaking the tie. There are five fibrosis stages, from F0 (no fibrosis) to F4 (cirrhosis or advanced scarring).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Due to the risk of analysis inconsistencies, the NASH space is starting to embrace digital pathology, Kemble says. Sagimet is working with Singapore-based digital pathology company HistoIndex in the Phase IIb FASCINATE-2 trial (NCT04906421) investigating TVB-2640. HistoIndex also previously worked with Novartis on its Phase II FLIGHT-FXR trial (NCT02855164) that studied tropifexor. US-based companies PathAI and CRO Summit Clinical Research are working together to develop AI-powered tools for liver pathology assessment.

That said, Kemble adds that digital pathology currently only supplements analysis done by pathologists, with FASCINATE-2 collecting data from both digital and pathologist readings. FASCINATE-2 would provide exploratory readout for digital pathology in human volunteers, with Sagimet having previously used this approach in murine models, he notes.
FASCINATE-2 has a primary endpoint of histological reduction of at least two points in nonalcoholic fatty liver disease (NAFLD) activity score (NAS). NAS ranges from 0 to 8, and covers steatosis, hepatocellular ballooning and lobular inflammation.
Sagimet asset has upstream NASH target
Sagimet’s oral candidate TVB-2640 works by inhibiting fatty acid synthase (FASN), which is a key enzyme related to fat synthesis. High fructose diet leads to excess production of palmitate, which leads to a metabolic cascade that can end up in NASH. Palmitate contains palmitic acid, which is the major type of fatty acid. TVB-2640 essentially blocks the upstream elements of what would trigger fatty liver disease, Kemble explains.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSagimet is scheduled to appear at the upcoming American Association for the Study of Liver Diseases (AASLD) meeting in November. It will present consolidated data from its Phase II FASCINATE-1 trial (NCT03938246), which recruited patients in the US and China.
The rationale behind teasing the data between the two countries is that there have been questions around the influence of diet and lifestyle in NASH, Kemble notes. Data so far shows TVB-2640 has a similar liver fat reduction impact at three months in the two distinct populations, he added. Sagimet worked with its China-based partner Ascletis Pharma on the trial.





