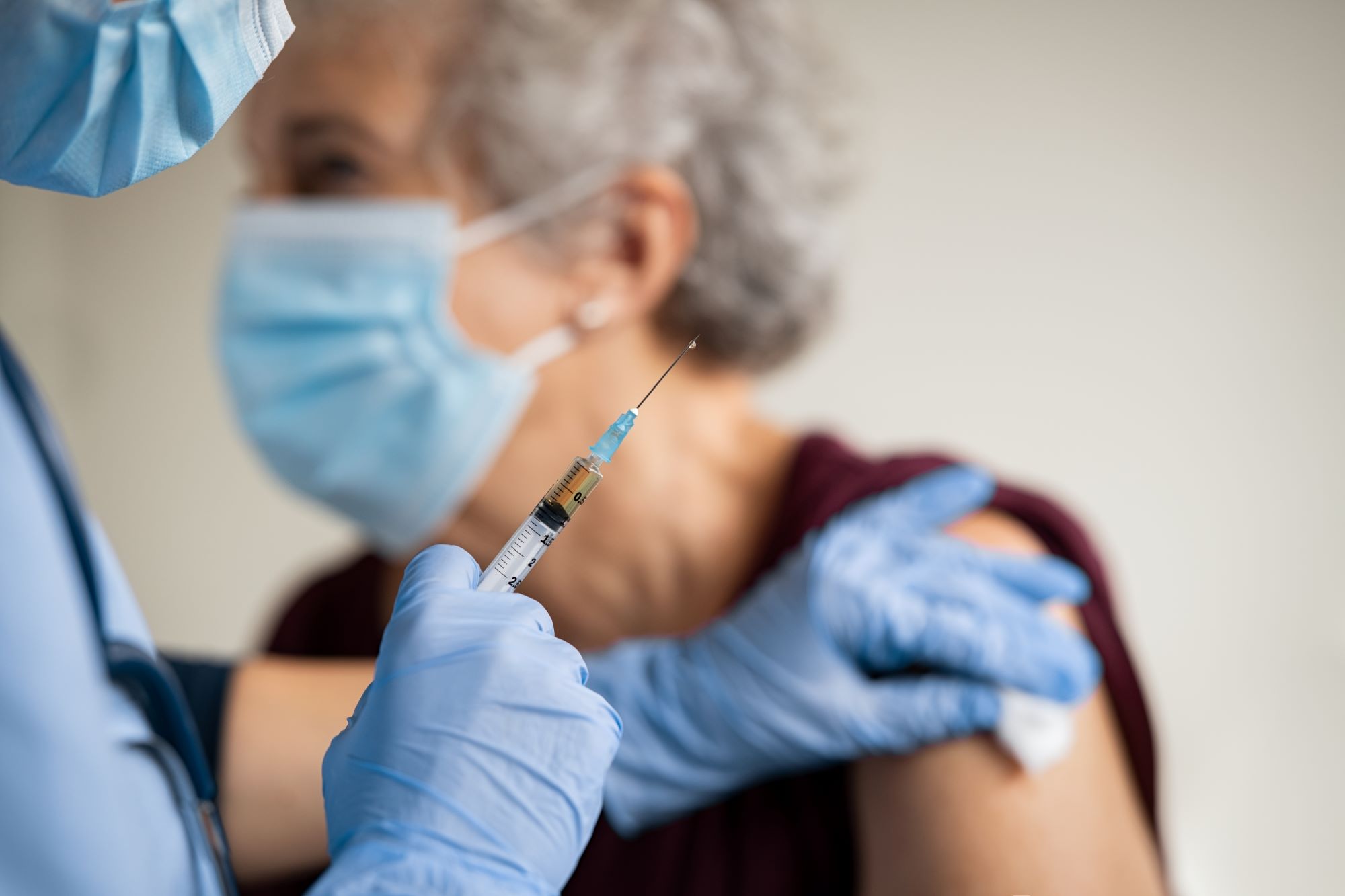
People, patients, participants – whatever you want to call the volunteers who take time out of their lives to help find cures and treatments, they represent the irreplaceable human resource that powers clinical trials. However, recruiting and retaining them is no simple task.
After all, being part of a clinical trial often requires a major time commitment and can be incredibly disruptive to a person’s life, especially if that person is sick with an illness that led them to a trial in the first place. Participating in clinical trials can also have hidden financial costs, from travel and accommodation to the cost of arranging childcare for participants who are parents.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Partly because of these burdens, patient recruitment and retention are two of the most challenging aspects of running clinical trials, and cost factors can also have an impact on trial diversity.
Paid participation can be beneficial to all parties
While paid participation is not a standard in clinical trials, there are sponsors and contract research organisations that do offer reimbursement for associated costs for their trial subjects. Major public research institutions often have the funding to compensate trial participants for their time – the National Institutes of Health (NIH) Clinical Center, the US’s largest hospital purely dedicated to clinical research, covers participants’ costs entirely, but that commitment is often not matched by smaller trial sites, while private sector trial compensation policies may vary. The issue is exacerbated in a highly privatised healthcare market like the US, where insurance coverage of treatments associated with clinical trials may not be a given.
In a recent guest editorial for USA Today, WebMD chief medical officer Dr John Whyte argued that the lack of a standardised approach to compensating trial participants is one of the main reasons that many trials struggle to meet recruitment targets, and that ethnic diversity in clinical research is so poor.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“The process is disjointed, and reimbursement levels are determined by drug and device companies sponsoring the research,” Whyte noted. “An independent review board, or IRB, then reviews the decision on payment and has the final say in how much a participant will receive, if anything, for each study.”
Clinical trials can pay between $50-$300 per day/visit, with compensation depending on the length of the time required as well as the procedures performed but some trials don’t pay anything or reimburse any costs.
Whether it’s lost income, travel expenses or childcare costs, offering compensation for the direct and indirect costs of trial participants would make clinical studies more accessible to a wider – and potentially more representative – patient population. It could also have knock-on benefits for trial sites and sponsors through a boost to patient enrolment and retention.
Paying participants could improve trial diversity
Over the past few years, a spotlight has been shone on the disparities in healthcare and the lack of diversity in clinical research.
People of colour (POC) are hugely underrepresented in clinical trials, even in trials for conditions that impact them far more than white people, such as cancer and diabetes.
The most recent multi-year report from the Food and Drug Administration found only 7% of clinical trial participants were Black and only 13% were Hispanic. “These shares are abysmal and haven’t changed in years, despite prolonged discussion and attempts to fix the problem that haven’t made an appreciable difference,” wrote Whyte, who believes paying participants would help level the playing field.
If ethnic minorities are more likely to experience financial hardship and therefore not be able to afford the associated costs of participating in a trial unless it is guaranteed that they will be reimbursed, it’s natural to assume that a more standardised approach to compensation could succeed in boosting POC representation in clinical research where community outreach and engagement has broadly failed to move the needle.
The ethical implications of paid participation
Of course, there is an ethical counter-argument when it comes to increasing the role of cash payments in the trial recruitment process. On paying and reimbursing subjects to take part in clinical trials, FDA guidance highlights the potential for ethical breaches around the coercive power of money: “Other than reimbursement for reasonable travel and lodging expenses, IRBs should be sensitive to whether other aspects of proposed payment for participation could present an undue influence, thus interfering with the potential subjects’ ability to give voluntary informed consent.”
“For too long, the FDA has taken a paternalistic approach to protecting patients from ‘unethical inducements’ to ensure they won’t be taken advantage of or coerced to participate,” Whyte argued in response. “But the argument is disingenuous. The reality is that if enrollees aren’t compensated for lost wages, travel, caregiving costs and any co-pays and deductibles that are not considered a routine trial cost by an insurer, then unfairness is already at work. There is nothing ethical about it.”
Indeed, there is a substantial difference between reasonable compensation for volunteers’ time and the kind of payments that might constitute an undue influence. But where does the boundary lie?
In a January 2020 research article entitled “When money talks: Judging risk and coercion in high-paying clinical trials,” Christina Leuker and co-authors wrote: “When clinical trials offer very high pay…some people consider them repugnant.”
To understand why, Leuker and colleagues asked 1,428 respondents to evaluate a hypothetical medical trial for a new Ebola vaccine offering three different payment amounts.
Some respondents (27%) saw very high pay (£10,000) as an indication that the trial posed potential risks. These respondents were also concerned that offering £10,000 was coercive – simply too profitable to pass up. Remuneration that is seen as coercive could of course lead to ethical issues and biases.
However, Whyte likens paid trial participation to compensating college athletes. “Pharmaceutical companies, universities, researchers and clinical trial sites all profit from the contributions of clinical trial participants, who literally give of themselves through blood, skin and tissue samples. Just like in college sports, everyone is benefitting except the people giving the most,” he wrote.
“Let’s not presume that the benefit to patients should simply be the ability to participate. It’s unrealistic to believe that people are going to enter clinical trials – often experimental medicines and invasive procedures – to make money. Patients don’t enrol in trials to get paid. They enrol to get better and live longer. When we don’t pay patients to participate in clinical trials, we limit enrolment.”
Read John Whyte’s opinion piece on USA Today here.



