Clinical trials, which are conducted in laboratories and designated centres, require the presence of professionals and constant monitoring of the enrolled subjects. Lock-downs and contagion fears have resulted in work from home or leave of absence for many amid heightened measures among healthcare workers.
The US FDA recommended switching to virtual patient visits than direct visits during the pandemic outbreak.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Verdict tried to find whether the coronavirus outbreak could cause delays in clinical trials, through a poll.
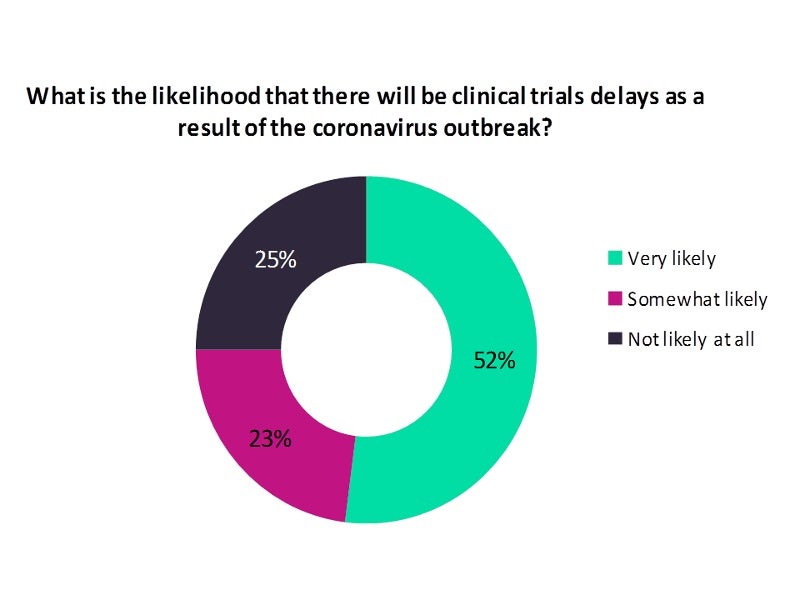
Run between 26 March and 02 April, the poll received 527 responses, the analysis of which revealed that clinical trial delays due to the outbreak are very likely, as polled by 52% of the respondents.
Delays are somewhat likely, according to 23% of the respondents, meaning that three-fourth of the poll respondents foresee clinical trial delays.
One-fourth of the respondents don’t expect the coronavirus outbreak to cause clinical trial delays at all, however.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData
Pharmaceutical companies are announcing the postponement of new clinical trials amid the outbreak, while few have announced delays to existing trials. Lilly, for example, stated that the timeline for its ongoing studies on mirikizumab, an experimental gastrointestinal treatment, would be delayed.
Another company Bristol paused the launch of new clinical trials for three weeks due to the ongoing pandemic.
Subdued clinical trial activity would result in revenue loss to hospitals and academic centres, in addition to the delays adding up to the cost of the drug developers.





