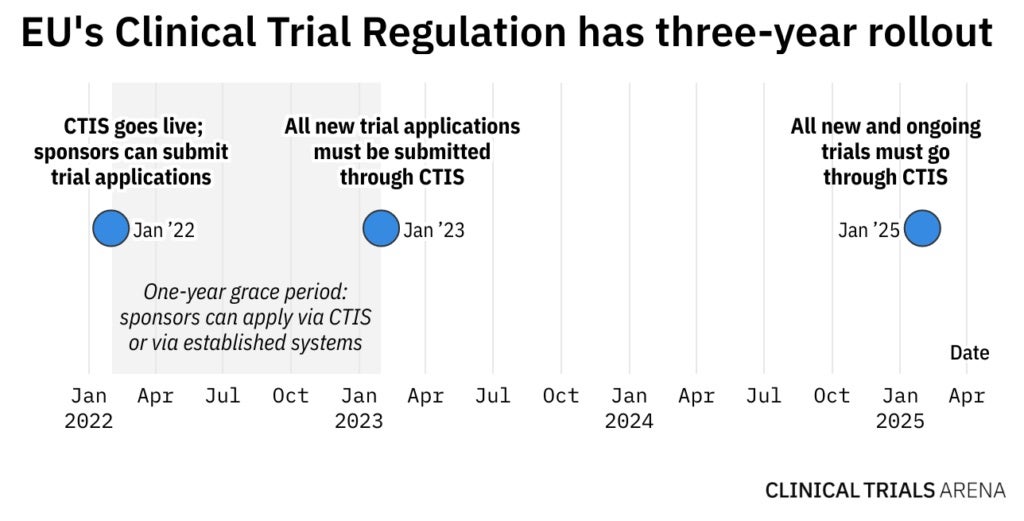
The year-long transition period is over. As of 31 January 2023, all new clinical trial applications (CTAs) in the EU/European Economic Area (EEA) will have to go through the EU’s Clinical Trials Information System (CTIS). This does not mean that EudraCT will completely shut down, as sponsors can still use it to manage ongoing trials that were registered in the old system. However, all new and ongoing clinical trials will have to go through CTIS from 31 January 2025.
According to the latest EMA’s Key Performance Indicator (KPI) report, the use of CTIS has been growing throughout the last year. In November 2022, 136 CTAs were submitted through the new system, which is the highest monthly number of applications so far.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Since the CTIS launch, 161 CTAs have been authorised and 204 CTAs are still under evaluation. The commercial sector is leading the overall number of applications and has the majority of authorised trials
CTIS journey so far
The EMA had an ad-hoc meeting with its Management Board earlier this month to discuss the recent improvements in the system and the progress made before the mandatory use of CTIS. The latest updates included the improvement and stabilisation of the user experience and the resolution of “80% of the blocking issues and related workarounds”.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThese technical issues are a part of the 2023 CTIS workplan and were raised during a board meeting, which took place in December 2022. The board will also review the current rules regarding the disclosure of certain trial documents and review CTIS transparency measures.
The uptake of CTIS saw some setbacks along the way. In July 2022, Clinical Trials Arena reported that target users were yet to jump in. At that time, sponsors with ongoing trials had little incentive to try the CTIS due to applications being approved under the old directive. Other complaints included tech-related issues.
Later in the year, the use of CTIS increased, yet the number of CTAs remained unsatisfactory. Experts told Clinical Trials Arena that some sponsors were hoping to complete their trials before the inevitable closure of EudraCT. The hesitation was also caused by a lack of awareness and understanding of the portal, as well as challenges in clinical trial data collection and management.





