Equillium has commenced a Phase I single ascending dose/multiple ascending dose (SAD/MAD) clinical trial of EQ102 in healthy subjects to potentially treat coeliac disease.
The company has concluded the dosing of eight subjects in the preliminary cohort while the dosing started in the second cohort.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The double-blind, randomised, placebo-controlled trial will analyse subcutaneous doses of EQ102 given as single or multiple doses in up to 64 subjects.
Part A will enrol healthy subjects, who will be categorised into five cohorts to receive single ascending doses of EQ102 or a placebo.
Healthy participants will be enrolled in Part B and randomised to three cohorts to receive EQ102’s multiple ascending doses or a placebo.
Evaluating the safety and tolerability of EQ102 is the primary endpoint of the trial.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAnalysing the pharmacokinetic and pharmacodynamic (PK/PD) changes are the trial’s secondary endpoints.
Following the conclusion of SAD/MAD segments, the company intends to assess EQ102’s biological activity in coeliac disease patients in Part C of the trial.
In patient-derived organoid cultures, EQ102 demonstrated to hinder IL-15 and IL-21 elicited signalling pathways in coeliac patient-derived intraepithelial cytotoxic T-Lymphocytes and crucial genes for tissue destruction.
The therapy was also found to avert intestinal tissue damage in a humanised mouse model of gastrointestinal inflammation in pre-clinical research.
The company intends to report preliminary data from the trial next year.
Equillium CEO Bruce Steel said: “This marks a major milestone as the first human study of EQ102, a therapeutic candidate generated de novo from our multi-cytokine inhibitor platform.
“EQ102 inhibits the natural biological synergy of cytokines IL-15 and IL-21 that drive cytotoxic T cell responses in gastrointestinal inflammation.”
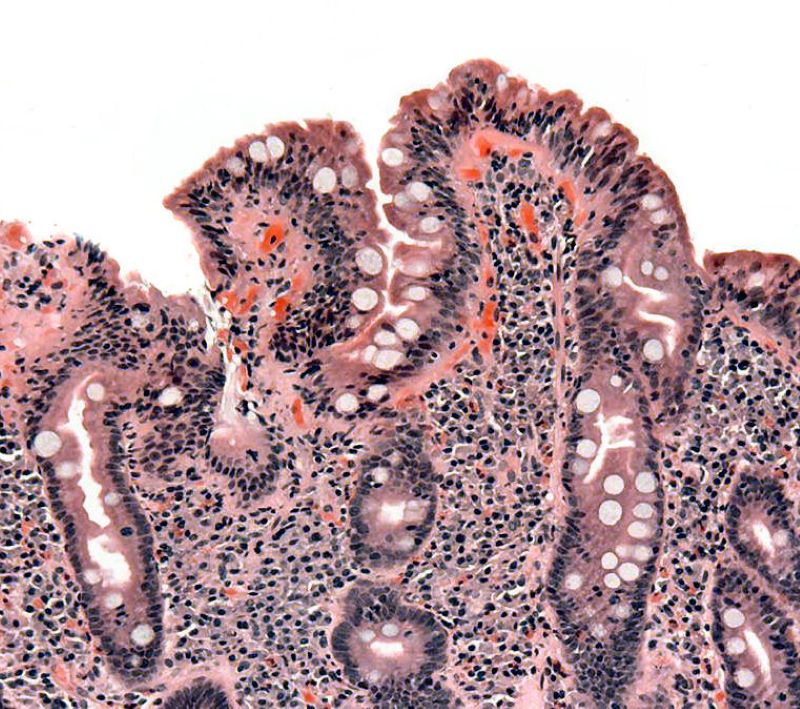
An inflammatory intestinal ailment, coeliac disease is caused by an inappropriate immune response to the dietary gluten consumption.
In March this year, the company began the Phase III EQUATOR trial of itolizumab for graft-versus-host disease.





