US subsidiary of Japan-based Eisai has initiated a large, randomised Phase III trial to compare the efficacy, safety and tolerability of eribulin mesylate injection to standard weekly paclitaxel as a first or second- line treatment for HER2-negative locally recurrent or metastatic breast cancer (MBC).
Patient enrolment is currently in process in the multicentre trial, which is being carried out in partnership with the Academic and Community Cancer Research United (ACCRU) group.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Around 910 people across the US are expected to be enrolled in the multicentre trial, which involves an investigational use of eribulin mesylate.
Eisai president of the oncology product creation unit Kenichi Nomoto said: "As a human healthcare company, Eisai is committed to further exploring new treatments for metastatic breast cancer patients."
The Phase III trial is intended for patients with HER2-negative breast cancer who have received no more than one prior chemotherapy regimen once their breast cancer has spread.
Eisai said that eligible patients will be randomly selected with equal allocation (1:1) to receive treatment for their cancer with either eribulin mesylate or standard weekly paclitaxel, within strata defined by prior adjuvant taxanes, hormone receptor status (ER/PgR), and line of therapy.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe trial’s primary endpoint is overall survival, while secondary endpoints include progression-free survival and objective tumour response rate.
Safety and tolerability of the treatment groups will also be continuously monitored in the Phase III study.
MBC is the form of the disease that refers to the most advanced stage, which cancer cells break away from the tumour in the breast, spread to other parts of the body and continue growing.
Eribulin mesylate injection, which is available as Halaven, is currently indicated for patients with breast cancer who have received at least two other types of medicines for their breast cancer once it has spread.
According to the company, Eribulin is a non-taxane, microtubule dynamics inhibitor that is a synthetic analogue of halichondrin B, a natural product that was isolated from the marine sponge Halichondria okadai.
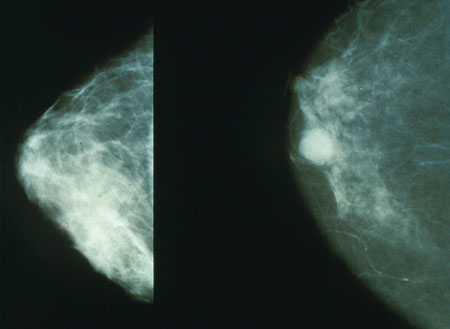
Image: Mammograms showing a normal breast (left) and a cancerous breast (right). Image: courtesy of Morning2k.





