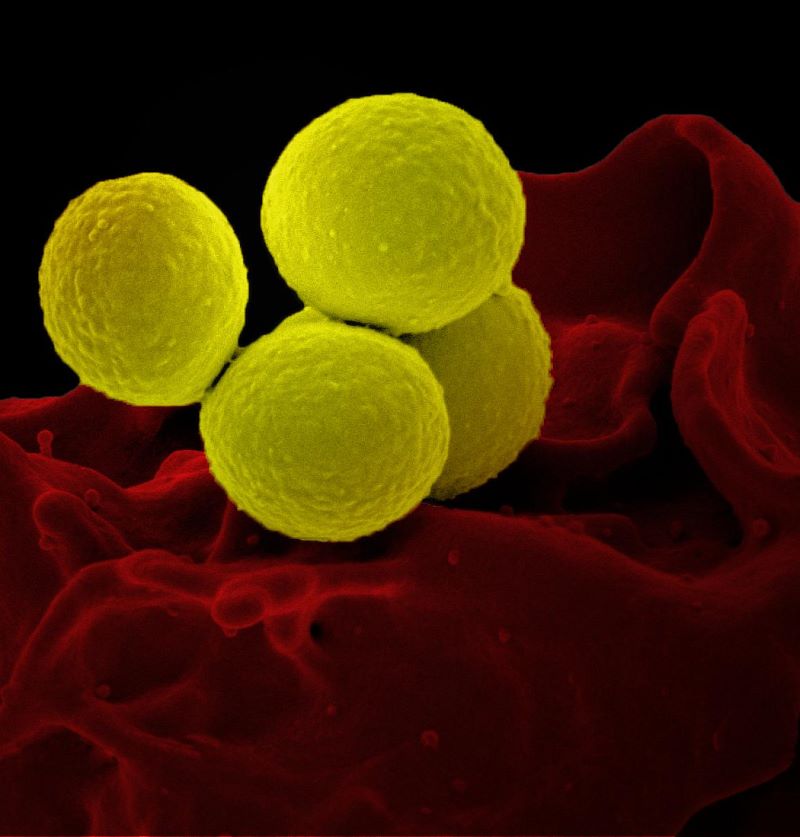
The National Institutes of Health (NIH) has announced the start of a Phase IIb clinical trial of existing antibiotic dalbavancin for treating complicated Staphylococcus aureus (S. aureus) bacteremia.
Dalbavancin is approved by the US FDA for treating acute bacterial skin and skin structure infections, including the S. aureus ones.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
With a strong activity against gram-positive bacteria, including methicillin-resistant S. aureus, the antibiotic could potentially be an effective treatment for S. aureus bacteremia.
S. aureus bacteremia is an infection of the blood and needs an invasive procedure of inserting a central intravenous (IV) catheter to provide long courses of antibiotics.
Named ‘Dalbavancin as an Option for Treatment of S. aureus Bacteremia (DOTS)’, the trial is sponsored by the NIH unit National Institute of Allergy and Infectious Diseases (NIAID) and carried out by NIAID-funded Antibacterial Resistance Leadership Group (ARLG).
It will enrol 200 adult subjects hospitalised with complicated S. aureus infection at approximately 20 sites in the US.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataIndividuals who have stabilised after initial treatment of their bacteremia will be eligible to take part in the trial.
The subjects will be randomised to receive the standard of care for complicated infections, including appropriate antibiotics or two intravenous 1500mg dose of dalbavancin given one week apart.
Various patient outcomes such as survival; additional complications (such as relapse) or clinical failures; drug-related adverse events; and overall quality of life will be evaluated at the end of the trial.
NIH noted that the regimen will have met the trial’s primary endpoint if subjects who received dalbavancin fare better on these metrics compared to those who received the current standard of care.
NIAID Director Anthony Fauci said: “As antibiotic-resistant infections become more widespread, better and easier treatment regimens are needed to ease the burden on both healthcare providers and patients.
“By investigating existing antibiotics for their action on a broader array of bacterial infections, we may be able to generate new treatment regimens more efficiently.”
Last week, the NIH announced plans to fund the Phase III ACTIV-6 clinical trial to analyse various prescription and over-the-counter medications that are currently available for self-administration to treat Covid-19 symptoms.





