Novartis and Medicines for Malaria Venture (MMV) have reported positive data from the Phase II/III CALINA trial of Coartem (artemether 5mg/lumefantrine 60mg) in babies weighing less than 5kg with acute uncomplicated malaria.
The Coartem formulation for infants and neonates met the primary pharmacokinetic (PK) endpoint in infants aged over 28 days (cohort 1). The companies were quick to add that the PK endpoint was also within the calculated interval for the neonates aged less than 28 days (cohort 2), however, the sample size was ‘too small for a conclusive statistical evaluation’.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Novartis and MMV stated that the new formulation has ‘good efficacy and safety’. The companies have also filed for regulatory approval with the data from the CALINA study.
The open-label, single-arm Phase II/III CALINA trial (NCT04300309) enrolled 38 babies weighing less than 5kg with acute uncomplicated plasmodium falciparum malaria. The study evaluated the new Coartem dispersible tablet formulation for infants and neonates, Coartem <5kg.
Coartem was developed by Novartis and MMV, with the former leading development and the latter providing scientific and financial support. In 2009, the US Food and Drug Administration (FDA) approved Coartem (artemether 20 mg/lumefantrine 120 mg) for treating adults with malaria.
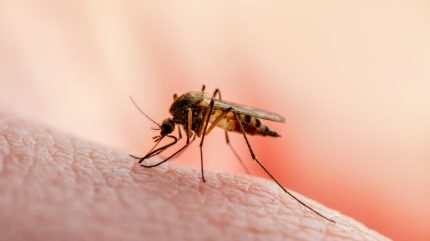
Malaria is an infectious disease caused by plasmodium falciparum protozoa, which is transmitted by infected female Anopheles mosquitoes. The 2023 WHO World Malaria Report estimated 249 million malaria cases and 608,000 deaths due to malaria in 2022.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMalaria is mostly found in tropical countries, with African regions having a high burden of the disease. Children under five years of age accounted for approximately 78% of all malaria deaths in the African region, as per WHO.
There has been renewed interest in developing treatment for malaria in recent years. In January, the UK Liverpool School of Tropical Medicine (LSTM) published data from the IMPROVE-2 study showing that the addition of the antimalarial drug dihydroartemisinin–piperaquine to daily co-trimoxazole substantially reduces the risk of malaria in pregnancy.



