A potentially life-saving drug for patients hospitalised with Covid-19 has been discovered by UK RECOVERY investigators at the University of Oxford.
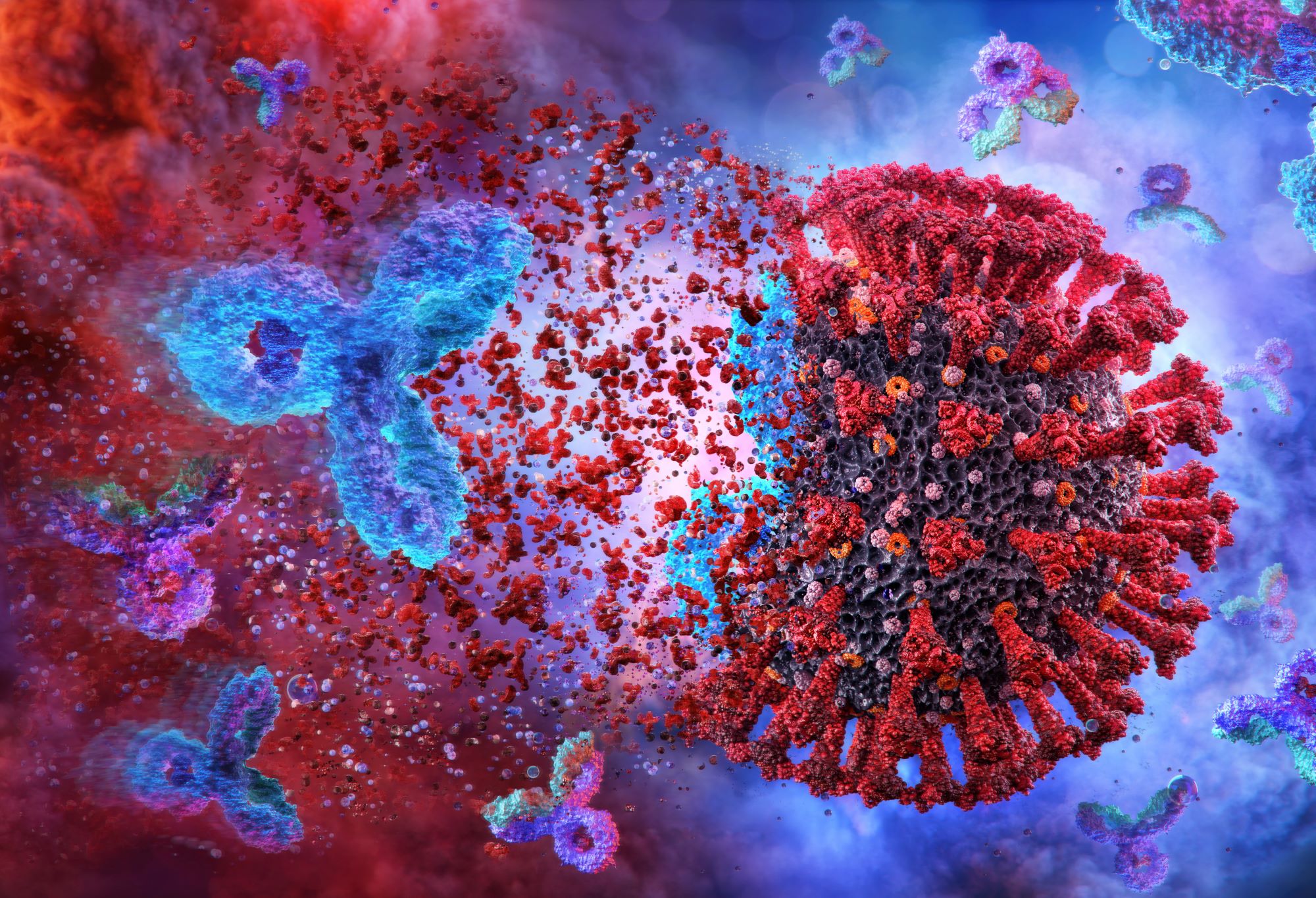
The researchers found that Regeneron’s REGEN-COV reduced the risk of 28-day mortality by 20% in patients hospitalised with Covid-19 who had not mounted their own immune response and tested negative for antibodies. These patients are known as seronegative.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
This translates to six fewer deaths for every 100 seronegative patients treated with the therapy.
The RECOVERY trial is the first to demonstrate that any antibody treatment improved survival in patients hospitalised with Covid-19 and the first large enough to definitively determine whether REGEN-COV specifically reduces mortality in this cohort.
It was conducted by the registered clinical trials units in the Nuffield Department of Population Health, in partnership with the Nuffield Department of Medicine, between September 2020 and May 2021.
The study involved 9,785 patients hospitalised with Covid-19 in hospitals across the UK. One-third showed no trace of producing their own antibodies. In this seronegative group, mortality was 30% among those who received standard clinical care but dropped to 24% among those given an intravenous infusion of 8,000mg of REGEN-COV.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“Definitive Phase 3 trials have now demonstrated that REGEN-COV can alter the course of Covid-19 infection from prevention, to very early infection, all the way through to when patients are on a ventilator in the hospital,” said Regeneron’s president and chief scientific officer George D. Yancopoulos.
REGEN-COV is a cocktail of two monoclonal antibodies, casirivimab with imdevimab, designed to block the infectivity of SARS-CoV-2. The two antibodies work by binding to two different points on the coronavirus spike protein, neutralising the ability of the virus to infect cells.
“These results are very exciting. The hope was that by giving a combination of antibodies targeting the SARS-CoV-2 virus we would be able to reduce the worst manifestations of Covid-19,” said University of Oxford professor of emerging infectious diseases and global health Sir Peter Horby, who was joint chief investigator for the RECOVERY trial.
“There was, however, great uncertainty about the value of antiviral therapies in late-stage Covid-19 disease. It is wonderful to learn that even in advanced Covid-19 disease, targeting the virus can reduce mortality in patients who have failed to mount an antibody response of their own.”
Previous Phase III trials in non-hospitalised Covid-19 patients demonstrated that REGEN-COV “reduced viral levels, shortened the time to resolution of symptoms and significantly reduced the risk of hospitalisation or death.”
In an earlier Phase 1/2 trial in hospitalised patients, REGEN-COV also swiftly reduced viral levels, with preliminary evidence indicating that it lowered the risk of death or receiving mechanical ventilation. Without REGEN-COV treatment, seronegative patients had higher mortality rates than patients who had already mounted their own immune response, who are known as seropositive.
“The RECOVERY trial has shown that in patients who had not made their own antibodies against SARS-CoV-2, treating them with REGEN-COV antibodies dramatically reduced their risk of dying or being on a ventilator, and also shortened how many days they remained in the hospital,” said Regeneron executive vice president and head of global clinical development David Weinreich.
“The trial was conducted at a time when most patients had not been fully vaccinated. These results provide hope to patients who have a poor immune response to either the vaccine or natural infection, as well as those who are exposed to variants for whom their existing antibodies might be sub-optimal.”
The therapy has already been granted emergency use authorisation for people with mild-to-moderate Covid-19 in the US. Regeneron has said that it will request that this authorisation be expanded to include appropriate hospitalised patients.
The results of the RECOVERY trial could pave the way for Regeneron’s antibody cocktail to be approved by UK regulators and rolled out for use across the NHS. The trial has already made several life-saving discoveries, including that the cheap and widely-used steroid dexamethasone was able to save lives among critically ill Covid-19 patients.
Not just Regeneron: Other possible treatments in the pipeline
Regeneron is not the only firm developing antibody treatments to help patients recover from Covid-19.
For the treatment of mild-to-moderate cases of Covid, US emergency use authorization has been granted to antibody treatments developed by Eli Lilly and Vir Biotechnology Inc with GlaxoSmithKline.
On Tuesday, AstraZeneca announced that its antibody cocktail had shown no evidence of protecting people from developing the disease following exposure, although other trials of its therapy as a prevention or a treatment are still underway.





