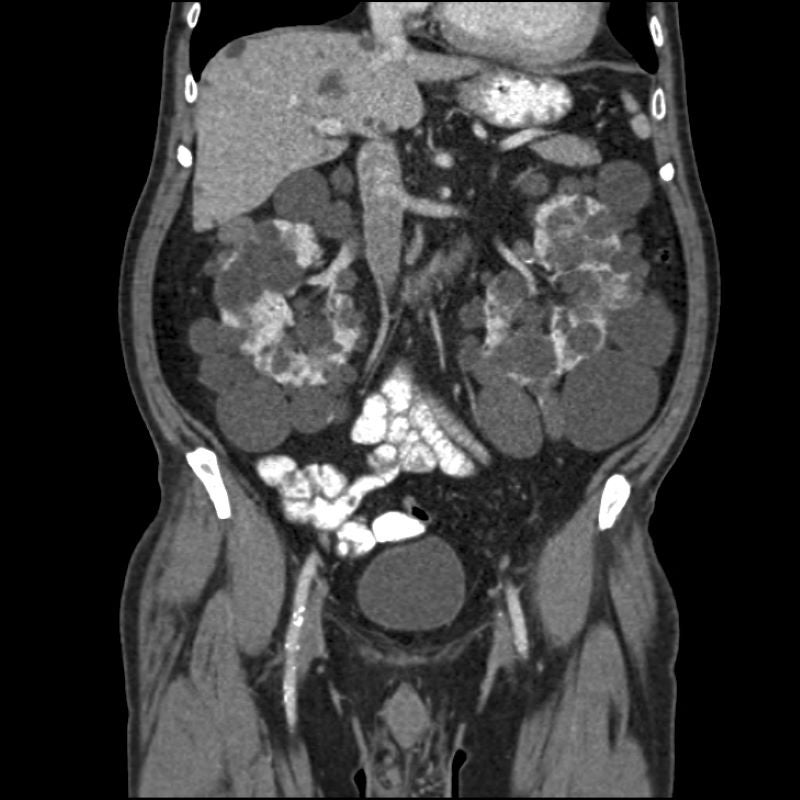
Regulus Therapeutics has reported positive topline findings from its Phase I single-ascending dose (SAD) clinical trial of RGLS8429 to treat autosomal dominant polycystic kidney disease (ADPKD).
In the trial, 32 participants received either RGLS8429 or a placebo.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
According to the findings, RGLS8429 was found to have a favourable safety and pharmacokinetic (PK) profile.
The treatment was also well-tolerated without any serious adverse events observed in the trial.
Nine mild adverse events (AE) were reported in the trial subjects, except sinus infections, which were found to be moderate in nature.
Plasma exposure was seen to be dose proportional for the four doses analysed in the trial, the initial data showed.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe levels were also comparable to the PK data from RGLS4326, a first-generation compound.
With the latest development, the company has commenced the placebo-controlled, double-blind Phase Ib multiple ascending dose (MAD) trial in ADPKD patients.
Evaluating the safety, tolerability, and PK of RGLS8429 is one of the trial’s primary objectives.
Analysing the efficacy of three varying dosages of RGLS8429, including variations in polycystins, cystic kidney volume (htTKV), and complete kidney function, will be the other primary objective.
The company plans to report topline findings from the first subject cohort of the trial in the first half of next year.
A new, next-generation oligonucleotide, RGLS8429 can hinder miR-17 and target the kidney.
Regulus Therapeutics president and CEO Jay Hagan said: “The safety and PK data generated in our Phase I SAD trial are highly encouraging and allow us the flexibility to use a range of dose levels, as we explore the safety and efficacy of RGLS8429 in the Phase Ib MAD study.
“We are excited about the initiation of the MAD study, and we look forward to presenting topline data from the first cohort of patients in the first half of 2023.”
In February last year, the company concluded subject enrolment in the first cohort of a Phase Ib clinical trial of RGLS4326 in patients with ADPKD.





