ACE-031 is an investigational protein therapeutic that is being developed by Acceleron Pharma for the treatment of Duchenne Muscular Dystrophy (DMD).
The drug received orphan designation from the US Food and Drug Administration (FDA) for treating DMD disease in August 2010.
On 4 August 2010, ACE-031 has received the US FDA’s Fast Track designation for the treatment of DMD.
On 9 September 2010, Acceleron and Shire entered into a strategic agreement to develop and commercialise the drug for treating DMD patients. As part of the agreement, Acceleron will get an upfront payment of $45m from Shire.
In addition, Acceleron will also get $165m and $288m in two stages from Shire towards royalty payments on product sales. Both the companies will collaborate on the worldwide development of the drug which is in its phase II clinical trial.
Duchenne muscular dystrophy (DMD)
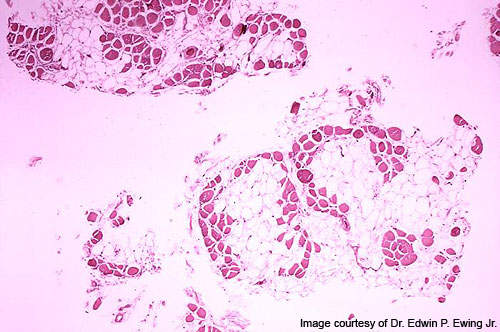
DMD is a fatal genetic disorder that is characterised by a gradual weakening of muscle strength and functions.
It occurs due to genetic mutations that can result in the absence or defect of a protein called dystrophin which is essential in maintaining the structural integrity of muscle fibres.
The deficiency of this protein leads to worsening of skeletal muscles and results in non-functional scar and fatty tissue.
DMD patients experience a relentless decline in muscular strength, which spoils their ability to breathe, walk and live independently. Most DMD patients get confined to wheelchairs and lose their upper body function.
The disease affects boys and occurs in 1 out of every 3,500 male births. It is estimated that there are more than 100,000 DMD patients in the US.
ACE-031
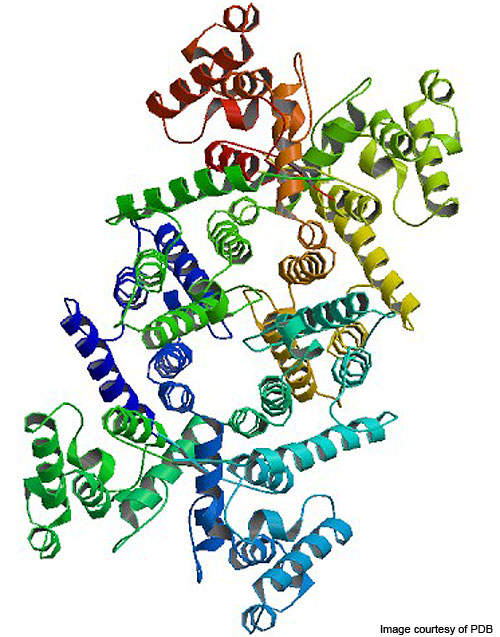
ACE-031 is a protein therapeutic. Muscle growth is regulated by proteins in the body which act as on or off switches. The drug contains a recombinant fusion protein which is produced by joining a portion of the human ActRIIB receptor to a portion of human antibody.
The muscles may become stronger when the drug acts by reducing the off signal which stops muscle production. The drug builds up the muscle and increases its strength by inhibiting a decoy receptor that binds myostatin before it is able to bind with active type II receptor (ActRIIB).
Clinical trials
Acceleron started Phase I clinical trials on ACE-031 in Canada in September 2008. The study enrolled 48 DMD patients and was concluded in July 2009. In the primary outcome measure the safety and tolerability of the drug were estimated by administering the drug in single doses of 1mg/kg and 3mg/kg.
After administering both placebo and single doses for 29 days, the muscle volume has increased by 3.5% when compared to 0.2% in placebo patients.
In the secondary outcome measure, pharmacokinetic (PK) and pharmacodynamic (PD) effects of the drug were evaluated.
Phase II clinical trials on the drug were started across multiple sites in Canada in April 2010 and are scheduled to conclude by February 2012. The study will enrol 76 boys with DMD over the age of four, who will be administered with an injection of one of four doses of the drug or a placebo once every four weeks for 12 weeks.
The primary outcome measure of the study is to find the safety and tolerability of ACE-031 by monitoring adverse events and clinical laboratory tests. The secondary outcome measures will be to check on change from the baseline in timed function tests and muscle strength tests.
Apart from testing the safety of the drug, the study will also evaluate the effects of the drug on muscle strength, muscle function and quality of life.
A type of whole body dual x-ray absorptiometry scan will be used to measure the increase in muscle size during the trial.
Acceleron plans to conduct clinical trials on the drug across many centres in the world. The company is in talks with many regulatory authorities regarding the possible expansion of the study.