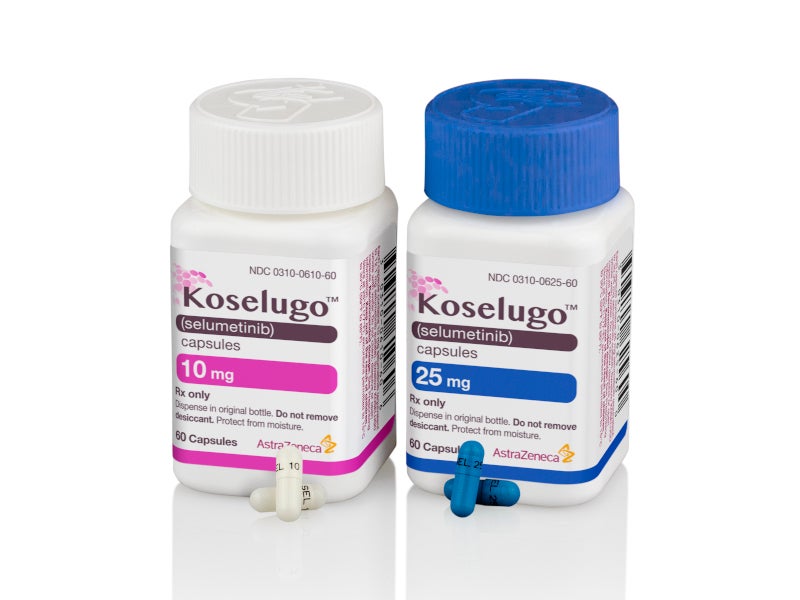
Koselugo® (selumetinib) is the first FDA-approved drug indicated for the treatment of neurofibromatosis type 1 (NFI), a rare and incurable genetic condition, developed and commercialised globally by AstraZeneca and Merck (MSD) under a licensing agreement.
The US Food and Drug Administration (FDA) approved the oral MEK inhibitor, Koselugo (selumetinib) for treating NF1 with symptomatic and irremediable plexiform neurofibromas (PN) in the paediatric patients of more than two years old in April 2020.
Koselugo (selumetinib) received EU orphan designation in August 2018 and orphan drug status (ODS) from Swissmedic in December 2018. It was granted FDA breakthrough therapy designation in April 2019 and rare paediatric disease designation in December 2019.
The US FDA accepted a new drug application (NDA) and granted priority review for Koselugo in November 2019.
AstraZeneca and Merck (MSD) submitted a marketing authorisation application (MAA) for Koselugo (selumetinib) to the European Medicines Agency (EMA) in early 2020.
A Prescription Drug User Fee Act (PDUFA) target action date is scheduled in the second quarter of 2020.
Koselugo (selumetinib) is available as oral capsules with a recommended dose of 25mg/m2 administered twice daily.
Neurofibromatosis type 1 causes and symptoms
Neurofibromatosis type 1 is a rare and untreatable genetic disease-causing irregular skin colour (pigmentation) and development of benign tumours on nerves, skin (neurofibromas), brain and other body parts.
NF1 is also known as Von Recklinghausen’s disease, peripheral plexiform neurofibromas (NF) and Von Recklinghausen neurofibromatosis.
Neurofibromatosis 1 is caused by mutations in the NF1 gene found on chromosome 17. The NF1 gene regulates the production of neurofibromin protein, which inhibits the uncontrolled tumour growth.
Common signs and symptoms include short stature, abnormal large head size (macrocephaly), skeletal malformations including a lateral curvature of the spine (scoliosis), speech difficulties, hyperactivity, high blood pressure and coloured bumps on the iris (Lisch nodules).
NF1 is mainly observed during the early years of childhood, anticipated to reduce life expectancy by up to 15 years.
Selumetinib mechanism of action
Selumetinib is a mitogen-activated protein kinases 1 and 2 (MEK1/2) inhibitor. The MEK1/2 proteins regulate the upstream extracellular signal-regulated kinase (ERK) pathway.
Both MEK and ERK are critical components of the RAS-regulated RAF-MEK-ERK pathway, often activated in various types of cancers.
Selumetinib suppressed ERK phosphorylation and reduced the number, volume and multiplication of neurofibroma in genetically modified mouse models.
Clinical trials on Koselugo
FDA approval of Koselugo (selumetinib; AZD6244; HYD Sulfate) comes from non-randomised, multicentre, open-label, single-arm phase 2 clinical trial, SPRINT Stratum 1.
The SPRINT Stratum 1 trial was coordinated by the NCI’s Centre for Cancer Research, Paediatric Oncology Branch and sponsored by the National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP).
In the SPRINT clinical trial, 99 eligible patients received selumetinib 25mg/m2 orally for every 12 hours on a daily basis during each 28-day cycle until unacceptable toxicity, patient withdrawal or disease progression.
The primary endpoint measure in SPRINT Stratum 1 trial was overall response rate (ORR), defined as the percentage of patients with confirmed complete response (vanishing of the target PN) or partial response of at least 20% reduction in PN volume on MRI that was confirmed on a subsequent MRI within 3-6 months.
ORR was 66% and all patients (n=33) witnessed a partial response and no patients had complete tumour disappearance while 82% of patients (n=27) had a response enduring for more than 12 months.
Serious adverse reactions observed during phase 2 SPRINT Stratum 1 trial were ocular toxicity, skin toxicity, increased creatinine phosphokinase (CPK), risk of bleeding, and foetal harm, cardiomyopathy, gastrointestinal toxicity and increased vitamin E levels.