Leqembi™ (lecanemab-irmb) is an anti-beta-amyloid (Aβ) monoclonal antibody (mAb) indicated to treat Alzheimer’s disease (AD).
Developed by Japanese pharmaceutical company Eisai, Leqembi must be administered only in patients who are in either mild cognitive impairment or mild dementia stage of the disease and have confirmed Aβ pathology. It is the second approved anti-amyloid therapy for AD after Biogen’s aducanumab.
Leqembi is available as a clear to opalescent and colourless to pale yellow solution in single-dose vials in 500mg/5ml and 200mg/2ml dosage strengths for intravenous infusion.
Development details of Leqembi
Leqembi (previously known as BAN2401) is the result of a collaborative research partnership established in 2005 between Eisai and BioArctic, a Sweden-based biopharmaceutical company. The primary objective of the alliance was to uncover an immunotherapy solution for AD. The research focuses on the Arctic mutation of amyloid beta-peptide, which was first identified by Professor Lannfelt at Uppsala University and is responsible for familial AD.
In December 2007, the companies entered into an exclusive licence agreement for BAN2401, under which Eisai obtained the global rights for the study, development, manufacturing, and commercialisation of the drug for the treatment of AD.
Eisai subsequently collaborated with US-based biotechnology company Biogen to jointly develop and commercialise BAN2401 in March 2014.
Eisai leads the development and regulatory submissions of lecanemab worldwide, with both Eisai and Biogen sharing the responsibility of commercialisation and promotion. The Japanese company holds the ultimate authority in decision-making for the product. BioArctic has the right to market lecanemab in the Nordic region under specific conditions and is currently collaborating with Eisai to prepare for its commercialisation in that area.
Regulatory approval for Leqembi
Lecanemab received breakthrough therapy designation from the US Food and Drug Administration (FDA) for the treatment of AD in June 2021.
In January 2023, the FDA approved Leqembi for the condition, under the accelerated approval pathway. It was followed by the submission of a supplemental biologics license application (sBLA) for Leqembi supported by the findings from Eisai’s global confirmatory Phase III clinical trial, Clarity AD, to secure complete approval for the drug under the traditional pathway.
Eisai also submitted a marketing authorisation application (MAA) for the drug to the European Medicines Agency (EMA) in Europe in January 2023, which was subsequently accepted in the same month. The Japanese Ministry of Health, Labour and Welfare (MHLW) granted priority review to the marketing application for the drug in the same month. Lecanemab is also under priority review in China.
The FDA accepted Eisai’s sBLA and granted priority review for the traditional approval of the drug in March 2023.
Leqembi’s mechanism of action
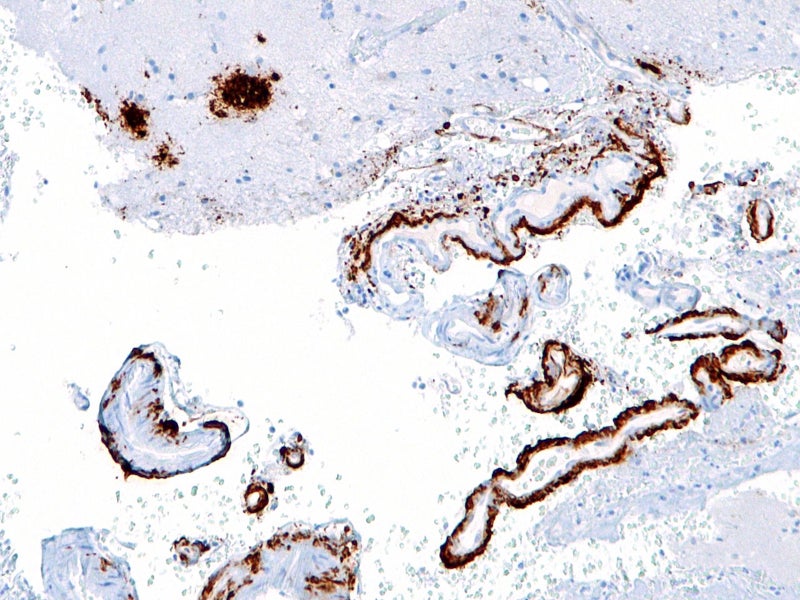
Lecanemab-irmb is a humanised immunoglobulin gamma 1 (IgG1) mAb directed against aggregated soluble and insoluble forms of Aβ peptide, the main component of the amyloid plaques found in the brains of individuals with AD. The accumulation of amyloid beta plaques in the brain is a significant pathophysiological feature of AD.
Lecanemab selectively binds to neutralise and remove soluble toxic Aβ aggregates that may potentially affect disease pathology and slow the disease progression.
Causes and symptoms of Alzheimer’s disease
AD is an irreversible, progressive brain disorder associated with a progressive decline in memory, thinking, and organising skills. Dementia is usually the most common cause of AD. Early symptoms of the disease include a cognitive decline in non-memory aspects such as getting the right word, trouble comprehending visual images and spatial relationships, and impaired reasoning or judgement.
The symptoms of Alzheimer’s get severe over time. Researchers believe the disease may start ten years or more before the first symptoms appear. AD can be of three stages: mild, moderate, and severe.
Symptoms may vary from moderate to severe as the disease progresses, with increased confusion and behaviour changes. The changes eventually affect the person’s ability to perform essential daily activities.
AD symptoms in general involve a gradual decline in memory, reasoning and handling of complex tasks, language, understanding visual form and space relationship, and behaviour and personality.
Clinical trials on Leqembi
The approval of Leqembi was based on the data from the extensive global Phase III confirmatory, double-blind, parallel-group, placebo-controlled Clarity AD clinical study.
The study enrolled 1,795 people with early AD aged from 50 to 90 years and randomised them to receive either a dosage of 10mg/kg of lecanemab every two weeks or a placebo.
The primary endpoint of the study was the change from baseline in the Clinical Dementia Rating-Sum of Boxes (CDR-SB) at 18 months.
The CDR-SB is measured in the range between 0 and 18, with higher scores indicating more significant impairment. The mean change of CDR-SB from baseline at 18 months was 1.21 and 1.66 for lecanemab and placebo groups, respectively.
The individuals treated with Leqembi showed a statistically significant reduction by -0.45 in clinical decline on the global cognitive and functional scale, compared with placebo at 18 months, representing a 27% slowing of clinical decline.
The most common adverse effects reported during the clinical trials were infusion-related reactions and amyloid-related imaging abnormalities with oedema or effusions. Other symptoms included headache, cough, and diarrhoea.