Discovered and developed by researchers at Pfizer, maraviroc is a novel treatment for infection due to the human immunodeficiency virus (HIV). A member of a new class of oral HIV drugs, maraviroc secured regulatory approval from the US FDA in August 2007. Marketed under the name Selzentry, it will be used for combination antiretoriviral treatment of adults infected with CCR5-topic HIV-1 detectable infection who have evidence of viral replication and have strains resistant to multiple antiretroviral agents.
Success in the US is likely to be mirrored in Europe, following the EMEAs decision to approve maraviroc (brand name Celsentri) for treatment-experienced adult patients infected with CCR5-tropic HIV-1 infection.
Pfizer’s maraviroc represents the first new class of oral HIV medicines to emerge in over 10 years.
HAART UNDERPINS TREATMENT OF HIV INFECTION
The advent of anti-retroviral drugs in the 1990s has had a major impact on the treatment of HIV. While unable to eliminate the virus from the body, these drugs nonetheless reduce symptoms, improve immune status, and delay the onset of AIDS. A combination regimen of highly active anti-retroviral therapy (HAART) is considered standard treatment for patients with HIV.
Ideally, it should be initiated before a patient’s CD4 cell count falls below 350–300 cells/ml. Typical regimens
include combinations of thymidine nucleoside analogues with a non-thymidine nucleoside analogue. The addition of a protease inhibitor or NNRTI to these regimens may improve outcome.
Although there are now a large number of approved drugs for treatment of HIV, the development of drug resistance can severely limit their clinical effectiveness. There remains an urgent need for new classes of drugs to combat HIV infection in the growing number of people who have become refractory to standard drug regimens.
CCR5 INHIBITORS REPRESENT A NEW CLASS OF HIV DRUGS
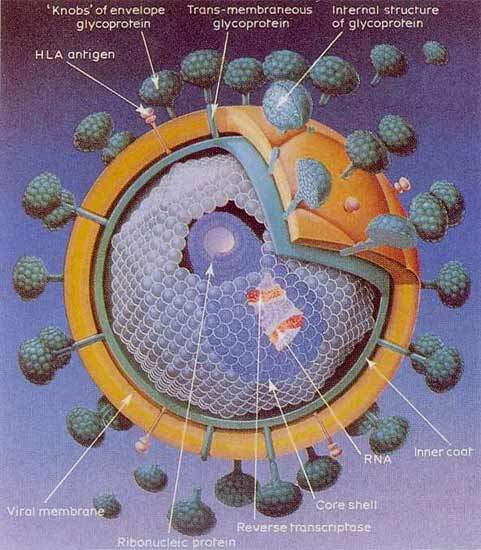
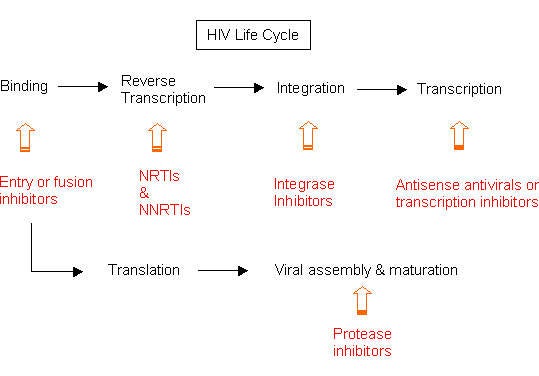
Maraviroc is a member of a new class of drugs called CCR5 inhibitors or CCR5 receptor antagonists. Unlike existing drugs for HIV, which target the virus inside white cells, CCR5 inhibitors are designed to prevent the virus from entering uninfected CD4-T cells – the target for HIV. These drugs act by blocking the CCR5 co-receptor on white cells, the route by which HIV enters cells.
In preclinical studies, maraviroc demonstrated activity against strains of HIV that use the CCR5 receptor to enter cells (so-called R5-tropic HIV-1), including multi-drug resistant strains.
MARAVIROC SIGNIFICANTLY REDUCES VIRAL LOAD
The clinical efficacy and safety of maraviroc has been investigated in the pivotal MOTIVATE (Maraviroc Plus Optimized Therapy in Viremic Antiretroviral Treatment
Experienced Patients) trials. In these trials, treatment-experienced HIV patients (n=1076) were randomised to treatment with maraviroc plus optimised background therapy or placebo plus optimised background therapy. Optimised background therapy consisted of three to six anti-retroviral agents, with or without low-dose ritonavir.
An analysis of interim data at 24 weeks from MOTIVATE trials 1 and 2 showed that almost twice as many patients in the maraviroc treatment arm achieved undetectable viral loads (<50 copies/mL HIV RNA) compared with patients in the placebo arm; mean reduction in HIV-1 RNA from baseline to week 24 was the primary endpoint in both trials.
In addition, patients receiving maraviroc-based treatment experienced an increase in CD4 cells that was nearly twice that of patients on the optimised regimen alone. All patients enrolled in these trials had CCR5-tropic HIV and viral loads above 5000 copies/ml HIV RNA on current treatment.
In these trials, the addition of maraviroc to optimised background therapy appeared safe and well tolerated. The frequency of adverse events in the maraviroc treatment was similar to optimised background therapy when adjusted for duration of exposure. Rate of premature discontinuation of therapy for adverse events was low (4–5%) and similar in the two treatment arms.
Safety is an important issue for this new class of drugs as concerns have been raised about potential risks of hepatic toxicity and neoplastic disease (lymphomas). Maraviroc is said to have shown no evidence to date of hepatic intolerance or concerns regarding neoplastic disease. There is also no evidence for progression of HIV in dual-tropic HIV patients.
MARKETING COMMENTARY
Since its discovery in the early 1980s, infection with HIV has reached epidemic proportions, especially in developing countries. Estimates suggest that 42 million people are living with HIV/AIDS, of whom around 3 million or more die each year. For HIV patients with access to treatment, the range of available therapies continues to grow. In the US the market for HIV drugs is expected to more than double by 2011.
CCR5 receptor antagonists represent an exciting new class of oral drugs for HIV with an entirely different mode of action to existing HIV therapies. Of the CCR5 receptor antagonists currently in development, Pfizer’s maraviroc is the first to market. It is understood that maraviroc will be offered in conjunction with a test developed by Monogram Biosciences, to determine whether a patient is infected with CCR5-tropic HIV and therefore likely to be a suitable candidate for treatment.