Semagacestat (LY-450139) was a gamma secretase inhibitor being developed as a treatment for Alzheimer’s disease by Eli Lilly. It was hoped that the drug would help to delay the onset of severe Alzheimer’s disease, and thereby help preserve cognitive and executive functioning and in turn improve patient quality of life.
In August 2010, Eli Lilly announced its decision to halt the development of Semagacestat. The decision was taken after analysing the preliminary results of the second Phase III clinical trial of the drug, which indicated that semagacestat failed to slow disease progression. The drug, in fact, worsened cognition and the ability to perform day-to-day activities.
Moving beyond symptom relief in Alzheimer’s disease
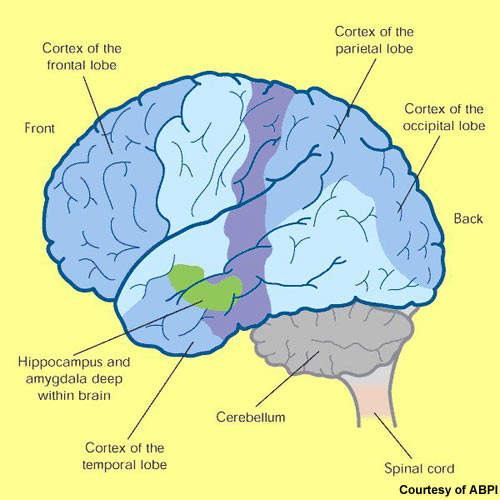
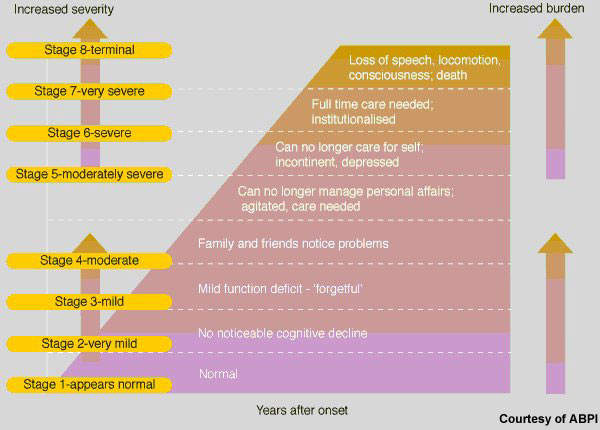
Alzheimer’s disease is a devastating neurodegenerative disease and the most common form of dementia to affect the elderly. From early symptoms of memory loss, the disease runs a progressive course of steady cognitive decline accompanied by severe behavioural problems.
Although specific anti-dementia drugs are available for patients with Alzheimer’s disease, they provide at best only modest symptomatic improvement. Moreover, their effects on cognition, executive functioning and behaviour are only temporary.
None is considered to have disease-modifying effects that can halt the progression of the disease and stop cognitive decline – the hallmark of Alzheimer’s disease. Many patients also fail treatment with first-line anti-dementia drugs because of poor efficacy and tolerability.
There is an urgent need to find drugs that can address the underlying pathology of Alzheimer’s disease and help halt the inexorable rise in the prevalence of the disease as the world’s elderly population rises.
Gamma secretase inhibitors target beta-amyloid
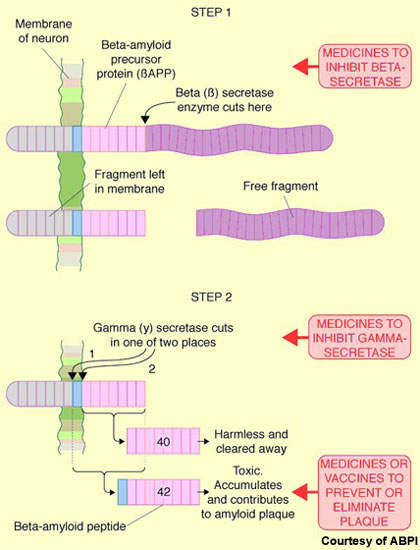
Pathologically, Alzheimer’s disease is characterised by the presence of amyloid plaques (neuritic or senile plaques) and neurofibrillary tangles in the brain. The amyloid plaques result from the accumulation of insoluble toxic beta-amyloid (b-amyloid42), derived from amyloid precursor protein (APP).
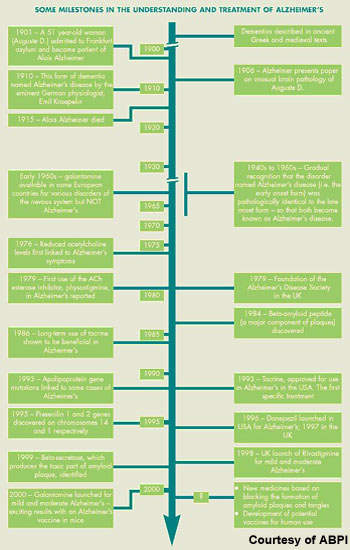
The production of toxic beta-amyloid is believed to trigger a series of secondary events, giving rise to the so-called amyloid cascade hypothesis. This has spurred the search for drugs to prevent b-amyloid42 formation.
Lilly’s semagacestat (LY-450139) is designed to inhibit gamma secretase, an enzyme that is involved in the cleavage of APP to beta-amyloid. By decreasing production of beta-amyloid, it is hoped that gamma secretase inhibitors will exert a disease-modifying effect in Alzheimer’s disease and thus slow or halt the destruction of nerve cells – the final stage in the amyloid cascade hypothesis.
Phase III IDENTITY trial
In March 2008 semagacestat (LY-450139) advanced to Phase III development, where it was evaluated in the IDENTITY (Interrupting Alzheimer’s Dementia by EvaluatiNg Treatment of AmyloId PaThologY) trial, the first Phase III trial for this new anti-dementia drug.
IDENTITY, an international multicentre randomised, double-blind, placebo-controlled trial, enrolled 1,500 patients with mild-to-moderate Alzheimer’s disease.
Integral to the design of this 21-month long study was a randomised delayed start, so that patients initially assigned placebo will receive active drug at some stage before the end of the double-blind treatment period. All patients completing the initial 21-month study period were eligible to continue open-label therapy.
Several different tests were used on a proportion of patients in the IDENTITY trial to measure the effect of semagacestat (LY-450139) on A-beta amyloid and amyloid plaques. For example, a new brain scan capable of taking images of amyloid plaques was used to measure the build up of amyloid plaques, while other tests will measure brain size and functioning. These additional tests helped to further determine the effect of semagacestat (LY-450139) on Alzheimer’s disease pathology.
Phase III IDENTITY 2 trial
In October 2008, Eli Lilly announced the start of the second Phase III study on semagacestat to understand what doses of semagacestat can be given in clinical practice. The study, IDENTITY 2, enrolled more than 2,600 people suffering from Alzheimer’s disease.
During the study period, patients were administered with either 140mg of semagacestat or placebo. Patients who were undergoing symptomatic treatment for Alzheimer’s disease were also also given semagacestat. The study included a ‘randomised delayed start’ design, which allowed even those patients who were initially given the placebo to take semagacestat before the trial period ended.
Solanezumab
In May 2009, Eli Lilly began two identical clinical trials on another anti-amyloid beta monoclonal antibody. Named solanezumab, the antibody is being tested as a potential medicine for the treatment of mild-to-moderate Alzheimer’s disease. The trial period will last for 18 months. Referred as EXPEDITION and EXPEDITION 2, the trials will enrol 2,000 patients above 55 years of age from 16 nations.
Marketing commentary
Recent estimates suggest that worldwide between 12 and 15 million people have Alzheimer’s disease, with about 5 million people affected in the US alone. Primarily a disease of the elderly, prevalence is projected to rise significantly as the proportion of elderly in the world’s population increases.
By 2050 there are likely to be more than three times as many people with Alzheimer’s disease than there are today, unless more effective treatments are discovered. While the search for curative Alzheimer’s disease therapies continues, any drug that can significantly address underlying disease pathology and slow disease progression would constitute a major advance.
Costs of care increase dramatically as patients advance to severe Alzheimer’s disease, when they are no longer able to function independently and often become bedridden.
Not surprisingly, the current market for anti-dementia therapies is in its infancy with among the highest growth dynamics in the CNS market.