Susvimo™ (ranibizumab) is a first-of-its-kind vascular endothelial growth factor (VEGF) inhibitor indicated for the treatment of neovascular (wet) age-related macular degeneration (nAMD) patients who have previously responded to at least two anti-VEGF injections.
Formerly known as Port Delivery System with ranibizumab, Susvimo is the first and only US Food and Drug Administration (FDA) approved therapy for nAMD that requires as few as two treatments a year.
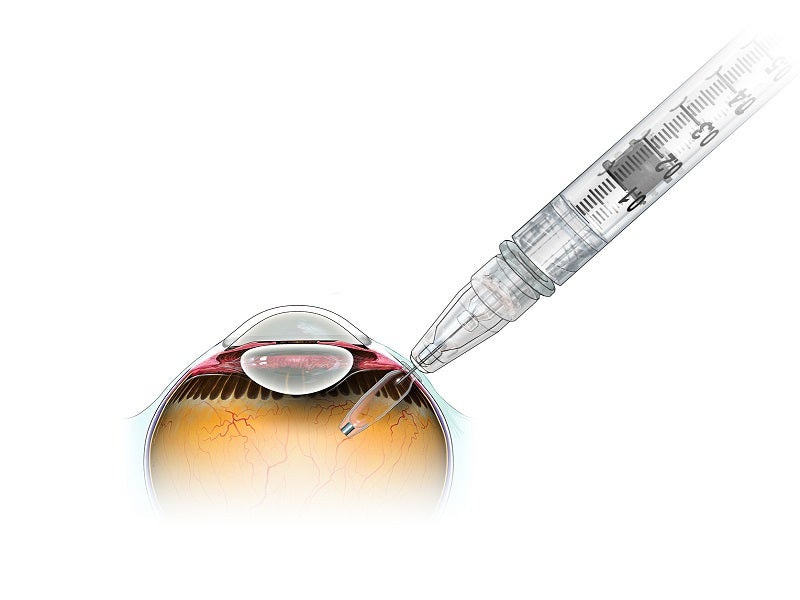
Susvimo is different from the FDA-approved Lucentis® intravitreal injection, although both have the same active ingredient ranibizumab. Susvimo’s port delivery system implant delivers the drug continuously for at least six months, managing patients’ wet AMD with just two treatments a year.
The Susvimo refillable ocular implant is surgically implanted in the eye by physicians. Over time, the implant consistently delivers a customised formulation of ranibizumab. Its refill-exchange procedures are also performed by the physicians under aseptic conditions.
Developed by Genentech, a member of the Roche Group, Susvimo is supplied in a single-dose vial as a sterile, clear to slightly opalescent, colourless to pale brown solution for intravitreal use via an ocular implant delivering 100mg/ml of ranibizumab.
Regulatory approvals for Susvimo
In April 2021, Genentech submitted a biologics license application (BLA) for Susvimo to treat patients with nAMD to the FDA. The BLA was accepted under priority review in June 2021.
The FDA approved Susvimo for the treatment of people with nAMD in October 2021.
Neovascular (wet) Age‑related Macular Degeneration (nAMD) causes and symptoms
Age-related macular degeneration (AMD) is a disorder that damages the macula, the portion of the eye that provides sharp, central vision critical for activities such as reading.
Neovascular or wet AMD (nAMD) is a more advanced form of the disease that causes vision to deteriorate rapidly, leading to vision loss. The development of abnormal blood vessels called choroidal neovascularisation (CNV) into the macula causes nAMD. CNV vessels leak fluid and blood that forms scar tissue and damages the central retina.
Wet AMD is the primary cause of visual loss in people aged older than 60 years, with around 20 million individuals suffering from the condition worldwide.
Signs and symptoms of nAMD include blurred vision, difficulties seeing from a distance or completing detailed work, blind spots developing in the line of sight, difficulty in differentiating colours, and edges and straight lines appearing wavy.
Susvimo’s mechanism of action
Ranibizumab is an intraocular recombinant humanised IgG1 kappa isotype monoclonal antibody fragment. It is a VEGF inhibitor that binds and inhibits the biologic activity of human VEGF-A, a protein that has proven to be important in both the development of new blood vessels and the leakiness of existing blood vessels.
Ranibizumab binds to VEGF-A and prevents it from interacting with its VEGFR1 and VEGFR2 receptors on the surface of endothelial cells, decreasing endothelial cell proliferation, vascular leakage, and the formation of new blood vessels.
Clinical trials on Susvimo
Susvimo’s clinical efficacy and safety for nAMD treatment were evaluated in a randomised, multi-centre, open-label, visual assessor-masked, active treatment-controlled Phase III Archway clinical study.
A total of 415 patients, including 248 in the Susvimo arm and 167 in the intravitreal ranibizumab arm, were randomised at a 3:2 ratio to receive either the continuous delivery of 100mg/ml Susvimo (ranibizumab) via implant every 24 weeks or 0.5mg intravitreal ranibizumab injections every four weeks.
Supplemental treatment with 0.5mg intravitreal ranibizumab injections was also available at specific weeks for patients who were randomised to the Susvimo arm if required.
The study’s primary outcome was the change in the Best Corrected Visual Acuity (BCVA) score from its baseline average over weeks 36 and 40.
Patients treated with Susvimo improved their visual acuity by an average of 0.2 eye chart letters from baseline, compared to 0.5 eye chart letters for the monthly ranibizumab arm, averaged over weeks 36 and 40.
Susvimo was generally well tolerated in the Archway study, with a favourable benefit-risk profile.
The most common adverse events (AEs) observed with Susvimo in patients during the clinical study were conjunctival haemorrhage (blood on the white of the eye), conjunctival hyperaemia (redness in the white of the eye), iritis, light sensitivity and eye pain.