VUMERITY™ (diroximel fumarate), previously known as ALKS 8700, is a novel oral formulation indicated for the treatment of relapsing forms of multiple sclerosis (MS).
The drug was jointly developed by Biogen and Alkermes under a licensing agreement signed in November 2017. Biogen holds the worldwide rights to commercialise the drug, while Alkermes will earn royalty on the net sales of the drug under the licensing agreement.
The partners submitted a new drug application (NDA) for VUMERITY to the US Food and Drug Administration (FDA) in December 2018. The drug received FDA approval in October 2019. Alkermes received $150m in milestone payment from Biogen in November 2019, following the FDA’s approval of the drug.
VUMERITY is available as delayed-release oral capsules with a starting dose of 231mg to be administered twice daily.
Multiple sclerosis causes and symptoms
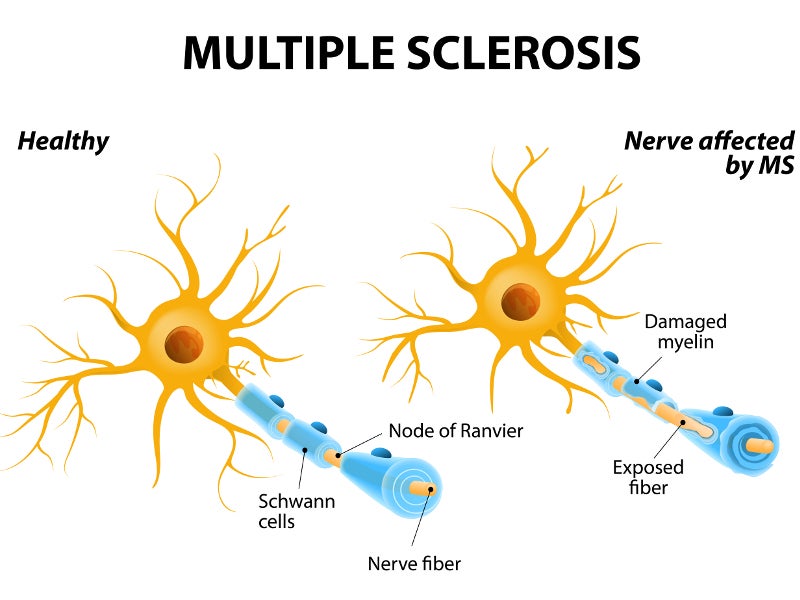
Multiple sclerosis is a lifelong condition that can affect the brain and spinal cord, causing potential disability in some cases. It is an autoimmune condition that damages and destroys myelin sheath, the protective layer around the nerves and the neurons.
The symptoms of multiple sclerosis include the inability to walk, muscle stiffness, blurred vision and problems with thinking and learning.
MS is one of the leading causes of disability in younger adults, categorised into two types, relapsing-remitting MS (RRMS) and primary progressive MS.
VUMERITY mechanism of action
The exact mechanism of action of VUMERITY is unknown. However, it is known to convert quickly into its active metabolite monomethyl fumarate (MMF) state upon administration. It activates the nuclear factor erythroid-2-related factor 2 (Nrf2) pathway both in-vitro and in-vivo.
The Nrf2 pathway is involved in the cellular response to oxidative stress, while MMF is identified as a nicotinic acid receptor agonist in-vitro.
Clinical trials on VUMERITY
The FDA approval of VUMERITY comes from pharmacokinetic bridging studies that compared the drug’s bioequivalence with TECFIDERA (dimethyl fumarate) in addition to safety and efficacy data of TECFIDERA from FDA findings.
The NDA submission also included data from EVOLVE-MS-1 study, which is part of the EVOLVE-MS (Endeavouring to Advance Treatment for Patients Living with Multiple Sclerosis) clinical development programme and includes EVOLVE-MS-2 study.
EVOLVE-MS-1 is an ongoing two-year phase three open-label study being conducted in patients with relapsing forms of multiple sclerosis to study the safety and tolerability of VUMERITY. EVOLVE-MS-2 is a multi-centre and open-label study being conducted in patients with RRMS to evaluate the gastrointestinal (GI) tolerability of the drug.
Safety and efficacy of the drug were established in two studies, study one and study two. A total of 1,234 patients were enrolled for the studies with a primary endpoint of the proportion of patients relapsed at two years.
Patients were given 240mg dimethyl fumarate twice or thrice daily or placebo for up to two years. The proportion of patients relapsing was 27% in the VUMERITY group, compared to 46% in the placebo group.
Study two was a randomised, double-blind, placebo-controlled study whose primary endpoints similar to those of study one. It included an open-label comparator arm in patients with RRMS. The patients were administered with 240mg dimethyl fumarate twice or thrice daily, an open-label comparator or placebo. VUMERITY displayed statistically significant improvement on the relapse rate in the study.
The most common adverse reactions observed in the trial were abdominal pain, diarrhoea, erythema, dyspepsia and lymphopenia.