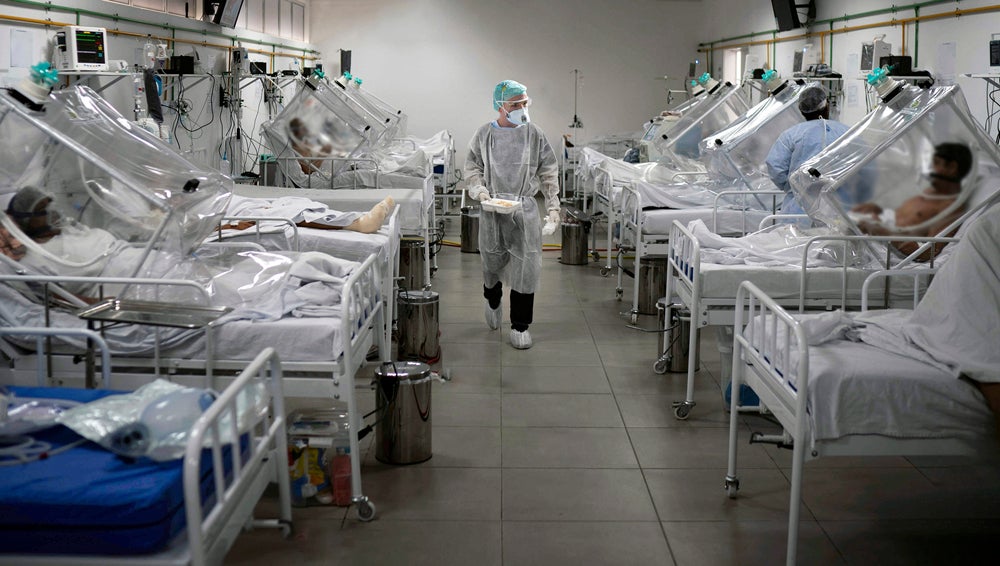
Cardiol Therapeutics expects to complete recruitment of its Phase II/III trial investigating its cannabidiol asset CardiolRx in hospitalised Covid-19 patients by the end of the year or in January 2022, CEO David Elsley says. Once its 422-patient enrolment target is reached, he notes the study would finish sometime in Q1 2022.
CardiolRx is an oral, cannabidiol-based approach that is not extracted from botanical sources but is pharmaceutically produced. Hospitalised Covid-19 patients go through what is referred to as a cytokine storm to defend itself against SARS-CoV-2. The rationale behind CardiolRx is that it would protect organs such as the heart, lungs, and others against this inflammatory response, Elsley explains.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The Phase II/III trial (NCT04615949) is enrolling patients at risk for, or with a prior history of, cardiovascular disease. The trial has coprimary endpoints of all-cause mortality, ICU admission and/or ventilator support, and cardiovascular complications – all three with a 28-day timeframe. Cardiol previously announced in December 2020 it is working with the contract research organisation Worldwide Clinical Trials in this study.
In recent months, there has been a resurgence of interest in Covid-19 therapeutics. Merck and Ridgeback’s recent data announcement for molnupiravir set the stage for its competitors in the mild-to-moderate disease space. In the moderate-to-severe Covid-19 market, there has been a slew of mixed clinical trial successes, with Gilead Sciences’ Veklury (remdesivir) and generic dexamethasone as reigning standards of care.
CardiolRx myocarditis trial imminent
Meanwhile, Canada-based Cardiol announced on 24 August it had secured clearance from the US FDA to commence a Phase II CardiolRx trial in myocardial recovery in patients with acute myocarditis. The trial is expected to be underway this quarter, Elsley adds.
The Phase II trial is expected to recruit 100 patients in the US and Europe and have coprimary endpoints ventricular function covering ejection fraction and longitudinal strain, as well as myocardial edema (extra-cellular volume). These endpoints will be evaluated after 12 weeks with either CardiolRx or placebo. Once the trial initiates, Elsley says it is expected to run for 9–12 months.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataPreclinical research shows that the cannabidiol molecule can reverse inflammation and fibrosis, Elsley explains. Inflammation of the heart muscle causes myocarditis, and when the heart is inflamed, scar tissue can manifest. Topline Phase I trial data shows CardiolRx has a favourable safety and tolerability profile, even at high doses, according to a 12 April media release.





