Developing and commercialising gene therapies can be a costly process. Specialised manufacturing facilities are required, and custom medicines can only be developed based on highly specific patient profiles. These do not come cheaply.
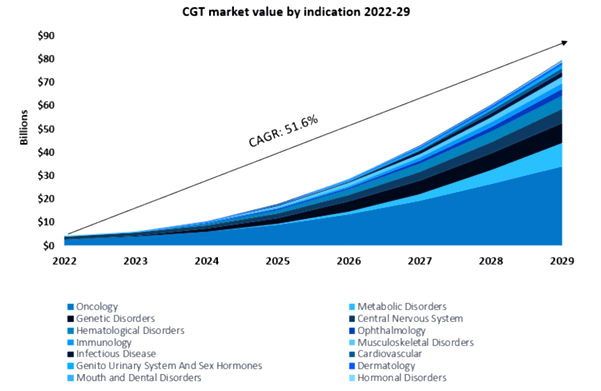
According to GlobalData’s Drugs database, there are currently over 4,450 gene therapies in preclinical and clinical trials. This indicates that gene therapies are continuing to gather pace; biomanufacturers have calculated that the rewards merit the risk. The projected growth of the gene therapy market is substantial, and GlobalData expects gene therapies to generate US$80 billion in sales by 2029[1].
As clinical trials ramp up in the realm of biologics, biomanufacturers must ensure robust purification processes. Originating from the production cell line, host-cell proteins (HCPs) are among the most complex process-related impurities. They can cause adverse reactions and significantly affect the quality, safety and efficacy of the final biologic drug. Detecting, quantifying and ultimately eliminating these impurities is critical.
For biomanufacturers looking to guarantee speed and safety in their clinical trials, HCP analysis can expedite and enhance purification processes. And a number of new products are hitting the market to make this analysis more efficient than ever before – all while keeping costs low.
ELISA kits
The host cells used for recombinant expression can contain thousands of HCPs capable of contaminating biopharmaceutical products. Stringent regulatory requirements place a burden on the manufacturer to reduce HCPs to the lowest levels possible. Failure to sufficiently remove impurities early in the drug development can mean manufacturers fall short of these requirements, hindering the product’s efficacy and potentially causing adverse patient reactions.
HCP ELISA kits are used to measure host cell proteins during process development and validation. Used to obtain relevant and precise data, ELISA kits can direct process improvement – giving biomanufacturers the tools they need to decrease levels of HCP contamination.
Only the most sensitive, specific and up-to-date HCP ELISA kits available should be used for this purpose. Working with an experienced partner is crucial for firms looking to amp up their anti-contamination strategies by leveraging industry-leading expertise.
A subsidiary of Maravai Life Sciences, Cygnus Technologies specialises in the development and supply of analytical products for the biotechnology and biopharmaceutical industries. The firm has pioneered a range of ELISA kits with proven sensitivity to downstream HCPs.
Cygnus offers generic HCP ELISA Kits for 21 different cell lines, with the company’s reputation for quality recognised by global regulatory agencies. Cygnus’ proprietary technology has been utilised in over 100 process-specific antibodies and immunoassays for leading biopharmaceutical companies around the world.
Antibodies and antigenic responses
An anti-HCP antibody specifically targets and binds to HCPs. In doing so, it offers a key tool for developing broadly reactive or ‘generic’ assays for detecting HCPs in process development and quality control. Customised, process-specific antibodies can be developed later as needed.
This is where the experience of a partner such as Cygnus really comes to the fore. Their tried-and-trusted methods of developing process-specific antibodies can help firms translate theory into action.
Cygnus can develop, qualify and manufacture robust kits that meet the specific process monitoring, release testing, and regulatory needs of its customers, as well as state-of-the-art orthogonal methods. This includes advanced antibody affinity extraction and mass spectrometry techniques – shoring up safety all while reducing risks, costs and time-to-market.
Host Cell DNA detection kits
Used to detect the presence of host cell DNA in a sample, DNA detection kits ensure the purity and quality of biopharmaceutical products – such as recombinant proteins and monoclonal antibodies – that are produced using host cells.
These kits are designed to be convenient, fast, and highly sensitive. They allow host cell DNA in products to be identified and eliminated at an early stage in development.
Cygnus host cell DNA detection kits utilise PicoGreen® DNA binding dye and include DNA extraction reagents and cell-line specific DNA calibrators. The Polymerase Chain Reaction (PCR) amplification kits contain DNA extraction reagents, DNA calibrators and validated specific primers.
Additionally, Cygnus DNA extraction kits employ a proprietary DNA extraction procedure that recovers <1 pg/ml residual DNA. They can also eliminate contaminating proteins, salts, and detergents from downstream DNA detection or amplification procedures.
Cygnus also offers reagents, DNA concentrates, and other host cell DNA kit components. This provides access to a comprehensive suite of tools and resources for all HCP analysis needs.
Other Process Related Impurity Kits
Purification or viral vectors for cell and gene therapies often involves affinity resins for AAV purification, bioprocessing enzymes (endonucleases, for example) to remove host cell nucleic acids and helper plasmids. In addition, growth media may include growth factors and other media additives. These proteins, enzymes, and purification resin leachates must be cleared to the lowest practical level. Cygnus offers ELISA kits that lead the industry in efficiency and sensitivity, enabling detection and quantification of these process-related impurities.
Cygnus Technologies is a pioneer in HCP analysis and has been helping biomanufacturers optimise purification processes for decades. Their expertise in Antibody Affinity Extraction (AAE), orthogonal methods of HCP analysis that integrate Mass Spectrometry (MS) and ELISA has made them a preferred choice for innovative pharma firms seeking to bring novel therapies to market. Download the whitepaper on this page to find out more.
[1] GlobalData Thematic Intelligence: The State of the Biopharmaceutical Industry 2024 Edition.


