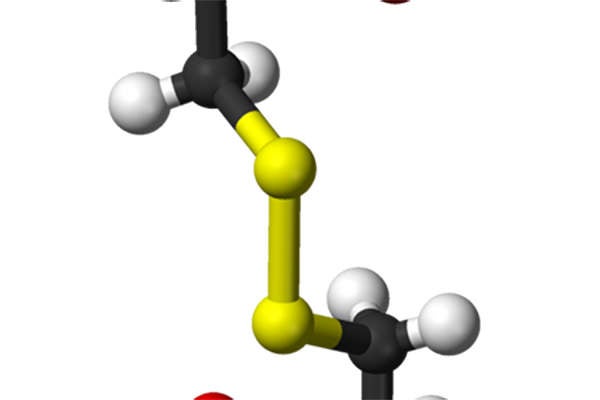
Procysbi (cysteamine bitartrate) is a delayed-release capsule indicated for the treatment of nephropathic cystinosis. The drug was discovered and developed by Raptor Pharmaceutical (Raptor).
Raptor received approval for Procysbi from the US Food and Drug Administration (FDA) in April 2013, for the treatment of adults and children who are aged six years and above suffering with nephropathic cystinosis.
Raptor has also submitted a marketing approval application for Procysbi in Europe. The drug is currently being reviewed by the European Medicines Agency (EMA), and is expected to be approved in the second half of 2013.
Nephropathic cystinosis causes
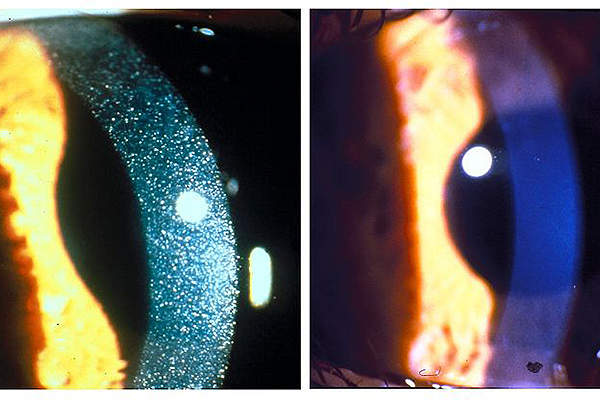
Nephropathic cystinosis is a metabolic lysosomal storage disorder that is genetically inherited. It causes accumulation of toxic cystine and can damage all cells, tissues and various organs in the body. It is a fatal disease that can lead to kidney failure, muscle wasting and blindness.
It is a very rare and orphan disease, which is estimated to affect nearly 500 people in the US and about 2,000 people worldwide.
Procysby’s mechanism of action
Procysbi contains a cystine depleting agent that is effective in preventing or delaying kidney transplants. The drug works by reducing the cystine content in cells and lowering the inherited defect of lysosomal transport.
The drug is available in capsule form for oral administration.
Clinical trials of Procysbi (cysteamine bitartrate)
The FDA approval for Procysbi was based on the results obtained from six Phase III clinical trials.
The first Phase III clinical trial was a single-centre, open-label, non-randomised two-period study. It enrolled nine patients with nephropathic cystinosis, who were aged between six and 24 years. The patients included eight children and one adult. The patients were administered with either immediate-release cysteamine bitartrate or Procysbi.
The study results showed that delayed-release Procysbi administered every 12 hours is as effective as immediate-release cysteamine bitartrate administered every six hours.
The second, fifth and sixth Phase III clinical trials of Procysbi were pharmokinetic studies that were conducted on the healthy volunteers.
The third Phase III trial of Procysbi was a pivotal, randomised, crossover and multicentre study that was conducted in the US and EU. It enrolled 43 patients aged between six and 26 years, of which 40 were paediatric and three were adult patients with nephropathic cystinosis. The patients were administered with either immediate-release cysteamine bitartrate or Procysbi.
The study results showed that the patients administered with Procysbi every 12 hours were non-inferior with respect to the depletion of white blood cell cystine levels, when compared to immediate release cysteamine bitartrate, which was administered every six hours.
The fourth Phase III clinical trial of Procysbi was an extension study of the third clinical trial, which was conducted for a period of 24 months. The study enrolled 14 children and four adult patients.
The results of the study showed that there was no average worsening of the kidney function reported with Procysbi extended treatment.
The adverse effects associated with Procysbi during the clinical trials included vomiting, abdominal pain, headaches, nausea and diarrhoea. The adverse effects also included anorexia, breath odour, fatigue, dizziness, skin odour and rash.
Marketing Procysbi in the US
Raptor is a leading biopharmaceutical company based in US. The company develops and commercialises therapeutics that treat debilitating and often fatal diseases. Procysbi is a leading product of the company.
Raptor entered into a $50m loan agreement with HealthCare Royalty Partners in December 2012, for the development and commercialisation of Procysbi. The early research of Procysbi was funded by Cystinosis Research Foundation.
The other medications available in the market for the treatment of same indication include Cystagon which is developed by Mylan Pharmaceuticals.