Moderna and Merck & Co have announced exciting data from the Phase IIb KEYNOTE-942 trial, which investigated a novel messenger RNA (mRNA) vaccine, known as mRNA-4157/V940, in melanoma patients. Moderna’s mRNA-4157/V940 and Merck & Co’s anti-(programmed cell death protein 1) PD-1 therapy, Keytruda (pembrolizumab), were given in combination as an adjuvant therapy following complete tumour resection in Stage III–IV melanoma patients. The adjuvant treatment of combined mRNA-4157/V940 and Keytruda cut the risk of recurrence or death by 44% compared with Keytruda monotherapy.
The trial, which enrolled 157 patients, reported no new safety concerns, with serious adverse events occurring in 14.4% of patients treated with the combination therapy versus 10% in patients treated with Keytruda monotherapy. In 2019, the FDA approved Keytruda monotherapy as an adjuvant therapy for Stage III melanoma patients on the basis of the KEYNOTE-054 study, with Keytruda resulting in 18-month recurrence-free survival of 71.4% versus 53.2% in the placebo arm. Adjuvant Keytruda is now utilised across all major pharmaceutical markets, with approval granted in more than 90 countries.
Historically, therapeutic cancer vaccines have demonstrated minimal efficacy. Educating the immune system to target genetically complex, rapidly evolving tumours that still present self antigens is a steep challenge. mRNA-4157/V940 can be described as a personalised vaccine that consists of a single mRNA molecule encoding up to 34 neoantigens, which are selected based on the individual tumour mutational signature. Once administered, the mRNA is translated and the resultant peptides are processed and presented, thereby stimulating an anti-tumour immune response. Keytruda was the first anti-PD-1 therapy to receive FDA approval for the treatment of advanced melanoma; immune checkpoint inhibitors (ICIs) are now the standard of care in this disease setting.
The anti-PD-1 monoclonal antibody (mAb) blocks the inhibitory interaction between PD-1 on T-cells and programmed death-ligand 1 (PD L1)/programmed cell death 1 ligand 2 (PD-L2) expressed on tumour cells, enabling a potent anti-tumour immune response. While Keytruda and other ICIs have revolutionised the outlook for patients with melanoma, a significant proportion of patients still fail to respond to this class of therapy. A combination of Keytruda with mRNA-4157/V940 primes the ‘unleashed’ immune system against tumours expressing the neoantigens incorporated in the mRNA vaccine. Plans to advance the combination into a Phase III trial and move into additional tumour types have been announced.
While this may be the first mRNA anti-cancer vaccine to demonstrate such promising efficacy, there are multiple mRNA vaccines currently in development for a range of oncology indications (Figure 1). Melanoma has more mRNA vaccines in its pipeline than any other tumour type, with several agents already in clinical trials, including BioNTech’s BNT-111 and Genentech and BioNTech’s BNT-122.
In contrast to mRNA-4157/V940, BNT-111 is an ‘off-the-shelf’ mRNA vaccine that encodes four melanoma-associated antigens that are expressed in more than 90% of cutaneous melanomas. Like mRNA-4157/V940, BNT-122 is a personalised mRNA vaccine, but it is limited to encoding 20 patient-specific neoantigens. BNT-111 is being investigated in the Phase II BNT111-01 trial in combination with Regeneron and Sanofi’s anti-PD-1 mAb Libtayo (cemiplimab-rwlc), while BNT-122 is being investigated in the Phase II IMCODE001 trial in combination with Keytruda.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData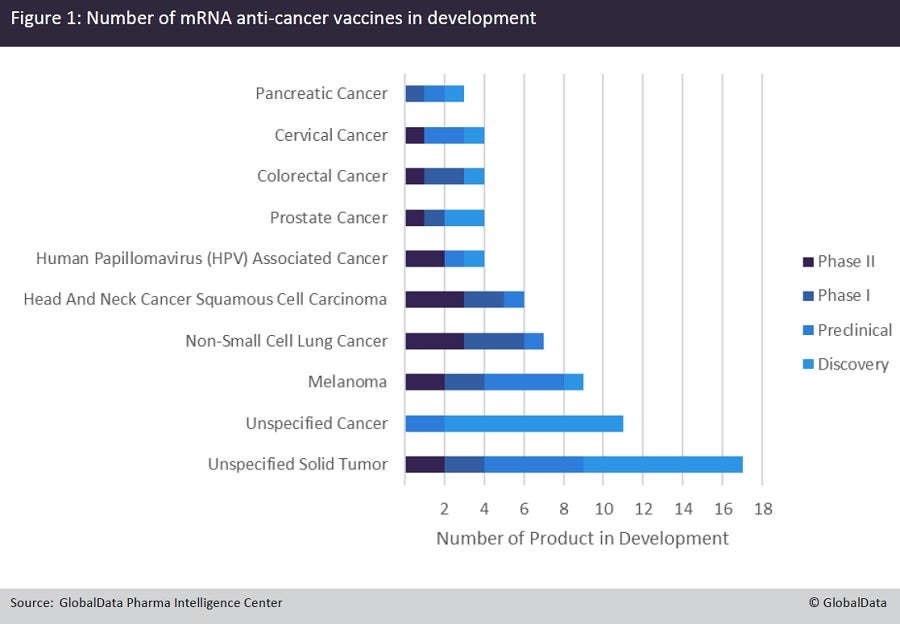
The use of mRNA vaccines for the treatment of cancer has become a promising approach, particularly when combined with ICIs to enable a more ferocious anti-tumour immune response. It remains to be seen whether a personalised therapy such as mRNA-4157/V940 is required for maximum efficacy, or whether an ‘off-the-shelf’ product can demonstrate equivalent efficacy. Personalised mRNA vaccines are likely to come with a hefty price tag and a relatively long manufacturing process, which will act as barriers to their therapeutic uptake.





