HERNEXEOS (zongertinib tablets) for the Treatment of HER-2 Mutant Advanced NSCLC, USA
HERNEXEOS® is indicated for the treatment of adult patients with unresectable or metastatic NSCLC.

Boehringer Ingelheim is one of the world's leading companies for contract development and manufacturing of biopharmaceuticals. All types of services from mammalian cell line or microbial strain development to final drug production and global market supply can be delivered within a one-stop-shop concept.
You have successfully submitted your enquiry. Someone from our company will respond ASAP
Boehringer Ingelheim is one of the world’s leading companies for contract development and manufacturing of biopharmaceuticals. All types of services from mammalian cell line or microbial strain development to final drug production and global market supply can be delivered within a one-stop-shop concept.
Boehringer Ingelheim has brought 19 molecules to market and has many years of experience in multiple molecule classes such as monoclonal antibodies, recombinant proteins, interferons, enzymes, fusion molecules, novel scaffold proteins and plasmid DNA.
At its large-scale manufacturing sites in Biberach (Germany), Fremont (US) and Vienna (Austria), Boehringer Ingelheim manufactures biopharmaceutical products under cGMP for the global market and for all stages of development. The Biberach and Fremont sites are specialised in highly efficient mammalian cell culture systems while the Vienna site offers high-yield bacteria and yeast processes for clinical and market supply.
For the past 125 years Boehringer Ingelheim’s basic principles and claim have remained to be outstanding in innovation and technology. It is our endeavour to bring your groundbreaking discoveries to the market with ever-improving methods for biomanufacturing.
Working with Boehringer Ingelheim brings a variety of benefits. A partnership with Boehringer Ingelheim enables both small and large-scale cGMP production in multi-product plants approved by the European, US and Canadian authorities. As an established privately owned and globally operating company, Boehringer Ingelheim is a reliable partner for regulatory agencies and can guarantee smooth registration proceedings. Regular audits by customers secure compliance with the latest standards and result in highest performance and good success rates.
Boehringer Ingelheim has broad expertise in multifarious biopharmaceutical customer projects and has experience in the shipment of cell lines, frozen and liquid bulk material and drug product, as well as vast knowledge in technology transfer and process adaptation.
The company’s contractual competence allows smooth and quick handling of all necessary agreements, and its reliable financial standing is important for long-term arrangements. Working with Boehringer Ingelheim enables accelerated entry to the market, portfolio and capacity enlargement, flexibility in project uptake supported by a global network of production partners, and many advantages thanks to the low costs of goods due to our balanced production programme.
Our biopharmaceutical production sites have the following capabilities and capacities:
We can offer you a wide range of services for the contract development and manufacturing of biopharmaceuticals. These include:
Our regulatory track record in the last ten years is excellent. We have successfully undergone in our multiproduct facilities more than 80 regulatory cGMP audits and pre-approval inspections (PAI) from the EMA, FDA, Health Canada, PDMA (Japan), tFDA (Taiwan), KFDA (Korea), GCC-DR (Gulf States), COFEPRIS (Mexico), ANVISA (Brazil), ISP (Chile), MOH (Kazakhstan), MOH (Russia), NDA (Uganda) and many regular customer inspections.
For approval of our customer biopharmaceuticals we support the regulatory documentation for the CMC section benefiting from our long-standing experience in IND, BLA and other regulatory filings.
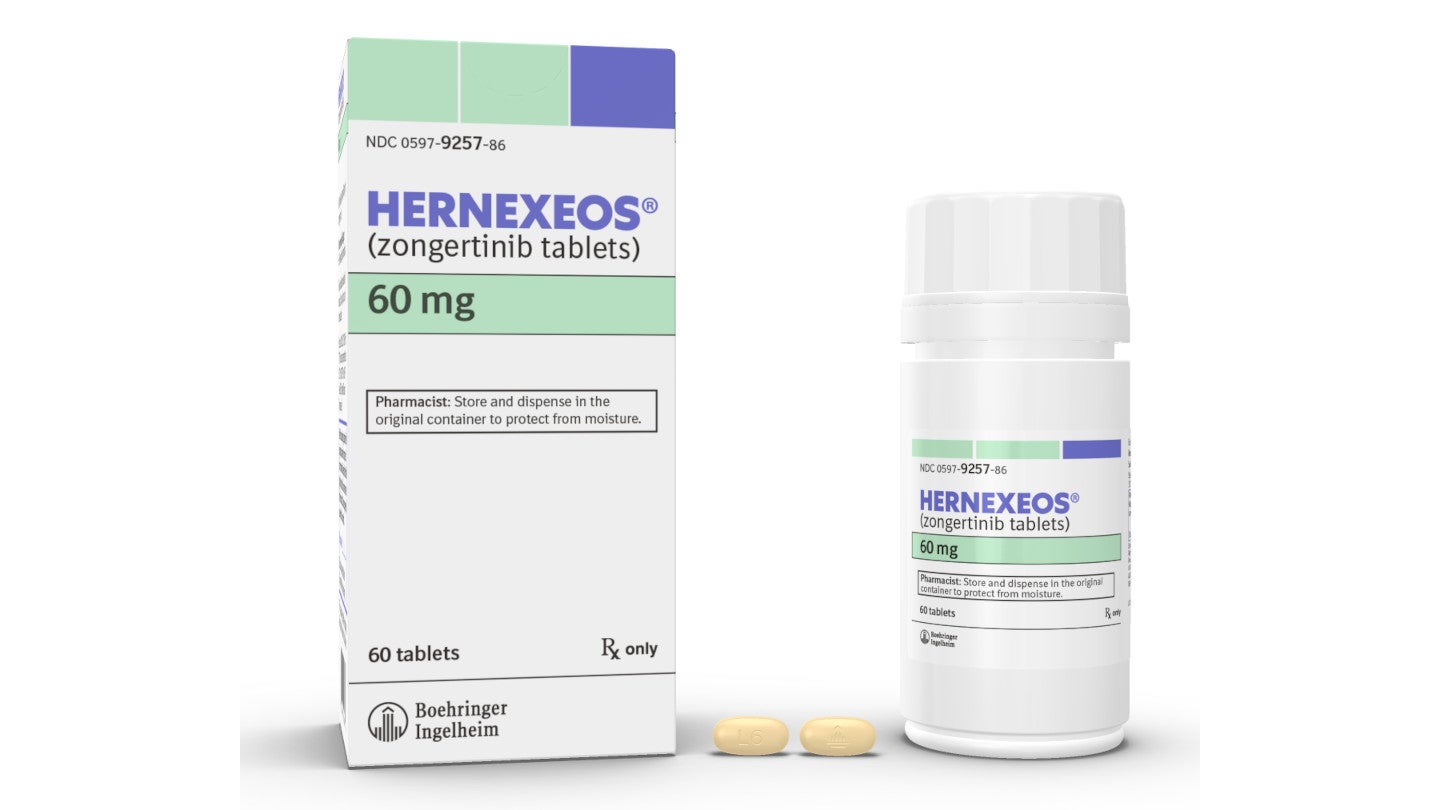
HERNEXEOS® is indicated for the treatment of adult patients with unresectable or metastatic NSCLC.
Developed by Sanofi, Soliqua 100/33 (insulin glargine & Lixisenatide injection) is indicated for the treatment of Type 2 diabetes.
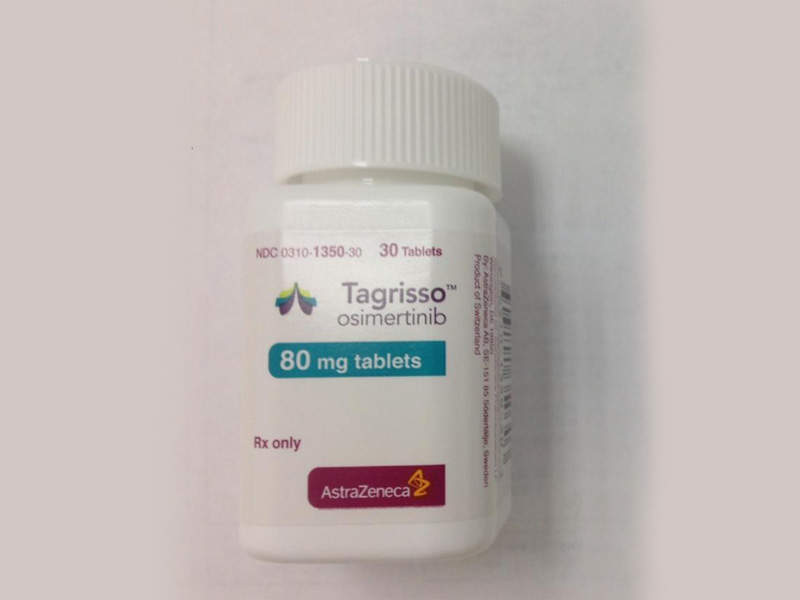
Tagrisso (osimertinib) is a once-daily tablet developed by AstraZeneca for the treatment of metastatic epidermal growth factor receptor (EGFR) T790M mutation associated with non-small cell lung cancer (NSCLC).

Xeljanz XR (tofacitinib citrate) is an 11mg once-daily oral JAK inhibitor developed by Pfizer for the treatment of moderate to severe Rheumatoid Arthritis (RA).
Anoro ellipta (umeclidinium bromide / vilanterol) is a dry powder for inhalation, indicated for the treatment of airflow obstructions in patients suffering from chronic obstructive pulmonary disease (COPD) or emphysema.