GRNOPC1 is an indicative stem cell therapy developed by Geron for the treatment of spinal cord injuries (SCI).
The US FDA approved the investigational new drug (IND) application of Geron in July 2010 and lifted the clinical hold on GRNOPC1 imposed in 2008. The FDA gave the go-ahead to Geron to conduct Phase I clinical trials on the drug for treating patients with acute SCI.
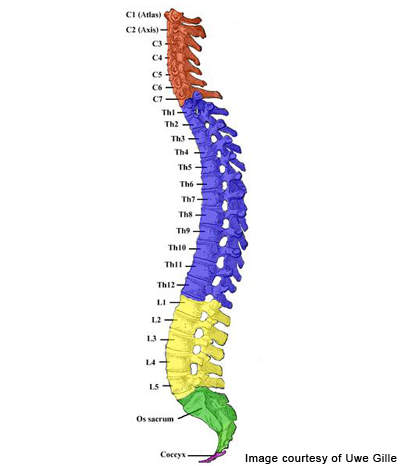
Spinal cord injury (SCI)
The spinal cord consists of many neurological segments. SCI begins with sudden damage to the spinal cord, resulting in paralytic functioning in mobility or feeling due to the damage of some neurons in the spine. The SCI results in the loss of oligodendrocytes that in turn affects myelin and neuronal loss which causes paralysis in many patients. The SCI is a medical emergency and prompt treatment using medicine and therapies can minimise the long-term effects.
In 2007, Christopher and Dana Reeve Foundation found that in the US alone about 1.3m were affected by SCI. The study also found that incidence of SCI is occurring 61% in males and 39% in females. The study also found that the average age for SCI is 48 years and the main causes include work-related accidents, motor vehicle accidents, falls and sporting accidents.
The incidence of SCI in the US is estimated to be approximately 12,000 cases per year and in China it is about 60,000 cases per year.
Targeting SCI with GRNOPC1
GRNOPC1 drug contains oligodendrocyte precursor cells (OPC), which are injected to SCI patients. The OPC will turn into oligodendrocytes to form myelin fatty sheath. Myelin is necessary for proper functioning of many of the nerves in the spinal cord and brain.
In SCI patients some neurons in the spine and its surrounding myelin get damaged. The drug treats or replaces only the surrounding myelin but not the neurons as it is necessary to improve myelin to stop further damage to the nerves.
Clinical trials
Preclinical studies of GRNOPC1 conducted on animals have shown that the drug is ineffective if administered after three months of injury.
With the US FDA having allowed Phase I clinical trials on GRNOPC1, Geron is planning to conduct multi-centre clinical trials on the drug to assess its safety and tolerability.
The study will be conducted by enrolling American Spinal Injury Association (ASIA) Impairment Scale grade A SCI patients.
The process for enrolling patients for Phase I clinical trials has been initiated.
The requisite for enrolment, however, is that the patients must have documented evidence of complete SCI with T3 to T10 spinal segments and the drug must be injected within 7 to 14 days following the spinal injury.
The primary endpoint of the trial is finding the safety aspects of the drug. The secondary endpoints will include assessment of efficacy of the drug such as sensation in the trunk or lower extremities or increased neuromuscular control.
Once the safety of the drug is established, Geron plans to approach the FDA for the approval of an extended study to increase the dose of the drug by enrolling a large number of ASIA Impairment grade B and C SCI patients.
For effective treatment it is necessary that GRNOPC1 must be precisely controlled while injecting into the lesion site. All the participating spine surgeons will be trained in needle placement and penetration, during the Phase I clinical trials.