HUMIRA® (adalimumab) is the only subcutaneous therapy approved by the US Food and Drug Administration (FDA) for the treatment of ulcerative colitis in paediatric patients aged five years and older.
The drug was developed by biopharmaceutical company AbbVie, which was founded in January 2013 by US-based healthcare company Abbott Laboratories as a spin-off of its research-based pharmaceuticals business.
HUMIRA is available in a single-dose pre-filled pen as a clear and colourless solution in 80mg/0.8ml, 40mg/0.8ml and 40mg/0.4ml dosage strengths for subcutaneous administration.
It is also available as single-dose pre-filled syringe in 80mg/0.8ml, 40mg/0.8ml, 40mg/0.4ml, 20mg/0.4ml, 20mg/0.2ml, 10mg/0.2ml and 10mg/0.1ml dosage strengths.
HUMIRA approvals
In February 2021, the FDA approved adalimumab, an anti-TNF (tumour necrosis factor) monoclonal antibody, for the treatment of children from five years of age with moderately to severely active ulcerative colitis.
Prior to the latest approval, adalimumab was approved in the US for the treatment of ten conditions, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, psoriasis, hidradenitis suppurativa, non-infectious intermediate, posterior and panuveitis, and juvenile idiopathic arthritis.
The drug was initially approved by the FDA in December 2002 for the treatment of patients with rheumatoid arthritis.
In September 2003, AbbVie Deutschland received marketing authorisation for the drug from the European Medicines Agency (EMA) for use on patients with inflammatory conditions in the European Union.
Ulcerative colitis causes and symptoms
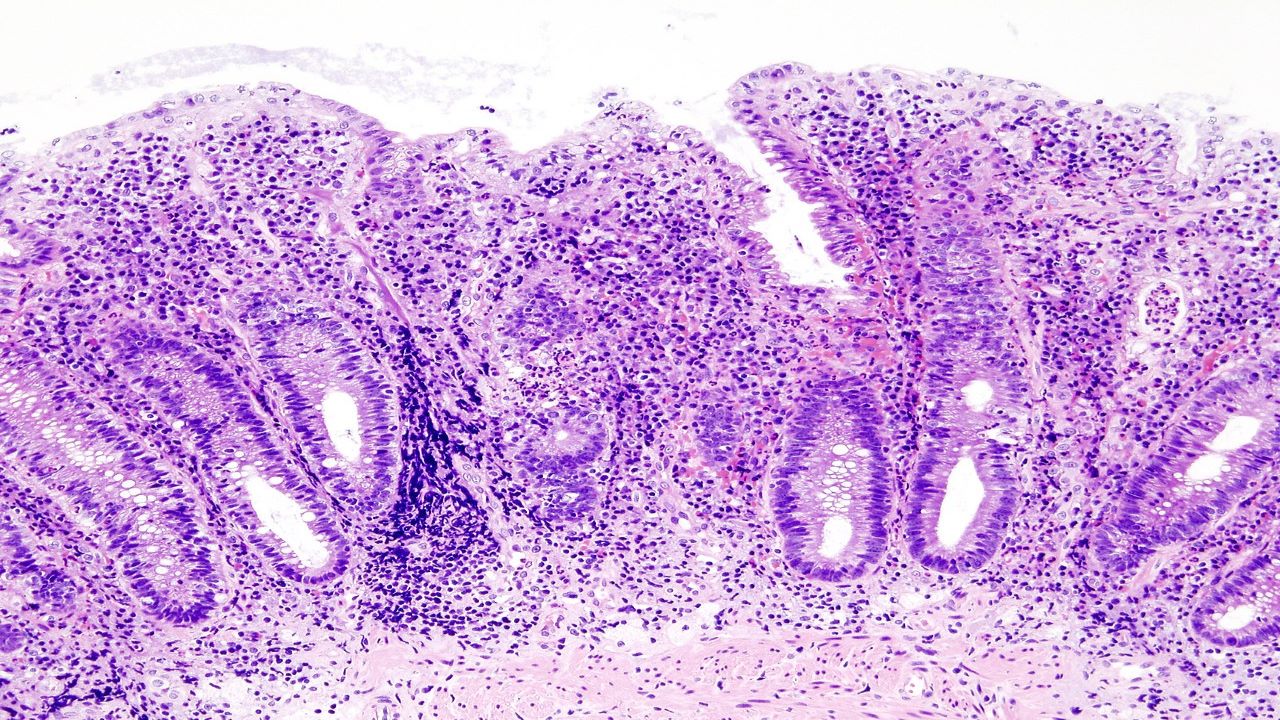
Ulcerative colitis is a chronic inflammatory bowel disorder characterised by inflammation of the large intestine.
The aetiology of ulcerative colitis is unknown, but research suggests that an abnormal reaction of the immune system causes inflammation and ulcers on the inner lining of the large intestine interaction. Ulcerative colitis is classified into four types, namely ulcerative proctitis, proctosigmoiditis, left-sided colitis and pancolitis.
Typical symptoms of the disease include abdominal pain, bloody diarrhoea, mild to severe bowel urgency, incontinence, weight loss and fatigue.
As children generally have more extensive disease and significant morbidity, there remains significant unmet treatment needs for children with moderate to severe ulcerative colitis compared to adults.
HUMIRA mechanism of action
The active substance in adalimumab is a monoclonal antibody designed to recognise and bind to TNF, a naturally occurring cytokine that plays an integral role in the inflammatory process and immune responses.
Adalimumab targets and blocks a source of inflammation by binding specifically to TNF alpha, an inflammation-causing protein, which helps to reduce the inflammation in the body that can lead to ulcerative colitis symptoms.
Clinical trials on HUMIRA
The FDA approval of the drug for ulcerative colitis in paediatric patients was based on the outcome of a phase three randomised, double-blind, multicentre study, ENVISION I.
The study evaluated the efficacy, safety and pharmacokinetics of adalimumab administered subcutaneously in child subjects with moderate to severe ulcerative colitis.
A total of 77 patients were given 2.4mg/kg (maximum of 160mg) of the drug at week zero, followed by 1.2mg/kg (maximum of 80mg) at week two and 0.6mg/kg (maximum of 40mg) at weeks four and six. A further 16 patients received open-label treatment with adalimumab at the higher dosage.
In the study, 62 patients who demonstrated clinical response per Partial Mayo Score (PMS) were randomised to receive a double-blind placebo, 0.6mg/kg (maximum of 40mg) of adalimumab, every other week, or every week between week eight and week 52.
The co-primary endpoints of the study included clinical remission per PMS at week eight, and clinical remission per Mayo Score at week 52 in patients who achieved clinical response per PMS at week eight.
Clinical remission was defined as a PMS or Full Mayo Score (FMS) less than or equal to two and no individual sub-score greater than one.
The clinical trial showed that treatment using adalimumab reached the co-primary endpoints of clinical remission at week eight and maintained remission at week 52 in patients who responded at week eight.
The most common adverse side effects reported during induction and maintenance periods included a headache and the worsening of ulcerative colitis.