ADYNOVI for the Treatment of Adults and Adolescents with Haemophilia A
ADYNOVI® is an intravenous infusion indicated for the treatment of adults and adolescents with haemophilia A.

Biotech companies rely on CROs and other service providers to take care of the operational aspects of clinical trials and ultimately achieve market approval, so finding a trustworthy and efficient outsourcing partner is one of the most vital aspects of running a successful clinical trial.
You have successfully submitted your enquiry. Someone from our company will respond ASAP
Biotech companies rely on CROs and other service providers to take care of the operational aspects of clinical trials and ultimately achieve market approval, so finding a trustworthy and efficient outsourcing partner is one of the most vital aspects of running a successful clinical trial.
The fourth annual Outsourcing in Clinical Trials New England conference will explore the latest and most important topics in the field of clinical outsourcing.
The fourth annual OCT New England will bring together respected industry experts such as:
Whether you are a start-up or an established company looking to enhance your existing outsourcing methodology, OCT New England can help you ensure the smooth completion of clinical trial procedures.
The event will take place 25-26 September 2012 at the Renaissance Boston Waterfront Hotel in Boston, Massachusetts, US. Sign up today and stay tuned as we bring you a superb line-up of guest speakers, exciting content and a room full of the latest technologies to enhance your clinical capabilities. Arena International Events and the OCT New England advisory board members look forward to seeing you in September for this must-attend event.
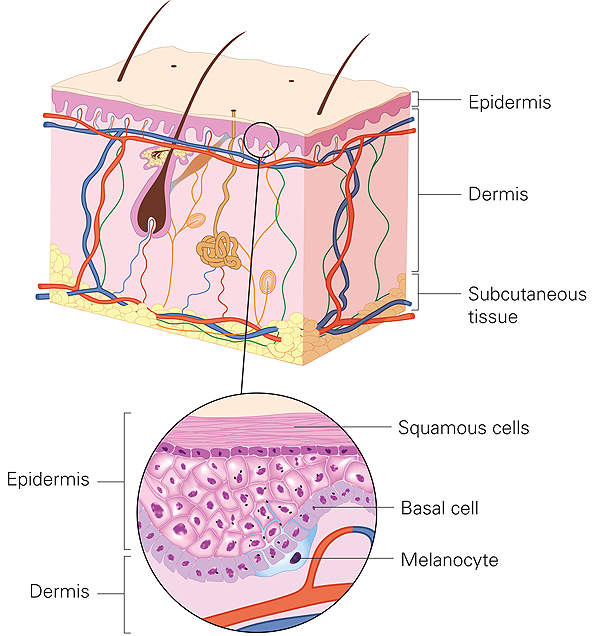
ADYNOVI® is an intravenous infusion indicated for the treatment of adults and adolescents with haemophilia A.
Hizentra® (immune globulin subcutaneous 20% liquid) is a subcutaneous infusion indicated for the treatment of patients with chronic inflammatory demyelinating polyneuropathy (CIDP).
Biktarvy® is a once-daily regimen indicated for the treatment of HIV-1 infection in adults that were previously not treated with anti-retroviral medicines.

Idhifa (enasidenib) is an isocitrate dehydrogenase 2 (IDH2) enzyme inhibitor indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukaemia (AML).
Nuwiq injection is the first recombinant anti-haemophilic factor (blood coagulation factor VIII) drug indicated for the treatment of patients with haemophilia A.
Abilify (aripiprazole), an orally administered dopamine partial agonist and a seratonin antagonist, was approved by US Food and Drug Administration (FDA) in November 2002 for the treatment of schizophrenia.