Lucemyra (lofexidine) for the Management of Opioid Withdrawal Symptoms
Lucemyra™ (lofexidine) is a non-opioid medication indicated for the mitigation of opioid withdrawal symptoms.

BioResearch Monitors is an independent provider of research-related quality assurance audit and compliance consulting services. Services include data quality and regulatory compliance, clinical quality assurance, GCP audits and good Laboratory practice services.
You have successfully submitted your enquiry. Someone from our company will respond ASAP
GCP quality assurance audits and regulatory compliance consulting for pharmaceutical research and development are the principal services offered by BioResearch Monitors. Since 1992, BioResearch Monitors has been a leading independent provider of research-related quality assurance audit and consulting services.
Our clients include major and emerging pharmaceutical and biotechnology companies, CROs and related organizations sponsoring and/or conducting worldwide research within the purview of the FDA’s Bioresearch Monitoring Program, ICH Good Practice guidelines and related multinational GxP quality standards.
BioResearch Monitors is dedicated to ensuring data quality and regulatory compliance in all phases of pharmaceutical research. The directors of BioResearch Monitors have been managing and conducting quality assurance operations since the inception of the FDA’s Bioresearch Monitoring Program. Our quality assurance associates are all highly qualified, with advanced degrees and significant, relevant industry and/or medical experience.
BioResearch Monitors prides itself on being flexible to meet the unexpected needs of its clients, and will do everything possible to meet tight project timelines and accommodate unplanned / unexpected ‘For Cause’ audits that need to be performed as soon as possible. BioResearch Monitors has conducted audits in many developing countries and is experienced in dealing with different cultures.
Clinical quality assurance audits, performed to ensure that scientific and ethical standards are met in the conduct of clinical trials, are conducted using proprietary computerized audit tools, customized to each project, so that GCP audit findings can be efficiently and consistently collected, analyzed and reported.
This focused systems-oriented approach to quality assurance is designed to assess and validate study conduct and documentation to ensure regulatory compliance and conformance to protocol, SOP and contractual requirements. Our methodology fully evaluates the activities and documentation underlying study data integrity and Good Clinical Practice (GCP) ethical and regulatory requirements.
To maintain independence and objectivity in performing quality assurance evaluations, BioResearch Monitors does not conduct or manage clinical trials, thereby providing our clients with unbiased opinions, free from any conflicts of interest, when sensitive investigations of internal systems or evaluations of other clinical service providers are needed.
GCP audit and consulting services for domestic and worldwide clinical quality assurance include, but are not limited to:
Our Good Laboratory Practice (GLP) services include sponsor and contract laboratory facilities inspections; critical phase inspections; SOP development and review; data and final report audits; and adjunct QAU functions.
Our Good Manufacturing Practice (GMP) services are principally related to clinical and nonclinical investigational products and include inspections of in-house and contracted drug product and drug substance manufacturing, packaging and labeling; process and scale-up validations; QA/QC systems evaluations; product complaint investigations; and logistics, storage and distribution audits.
Please call BioResearch Monitors to discuss how our quality assurance programs and services can add value and confidence to your research projects.
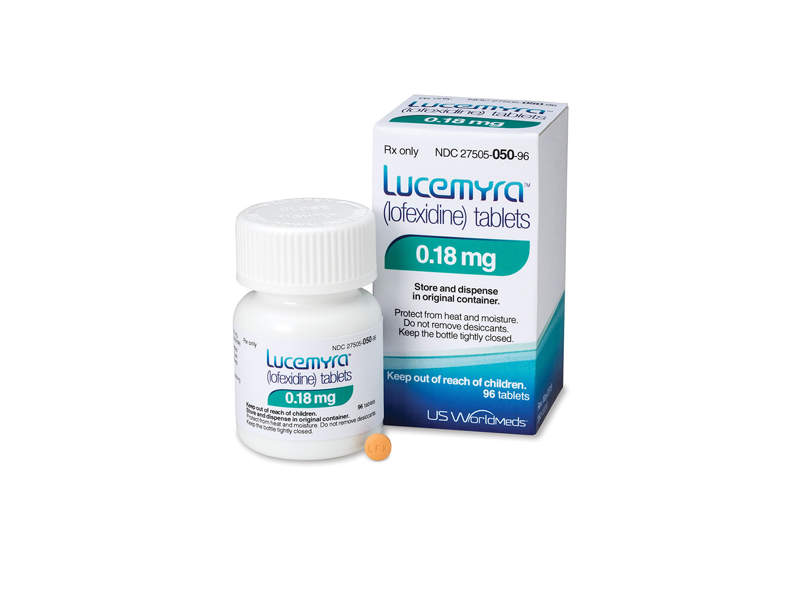
Lucemyra™ (lofexidine) is a non-opioid medication indicated for the mitigation of opioid withdrawal symptoms.
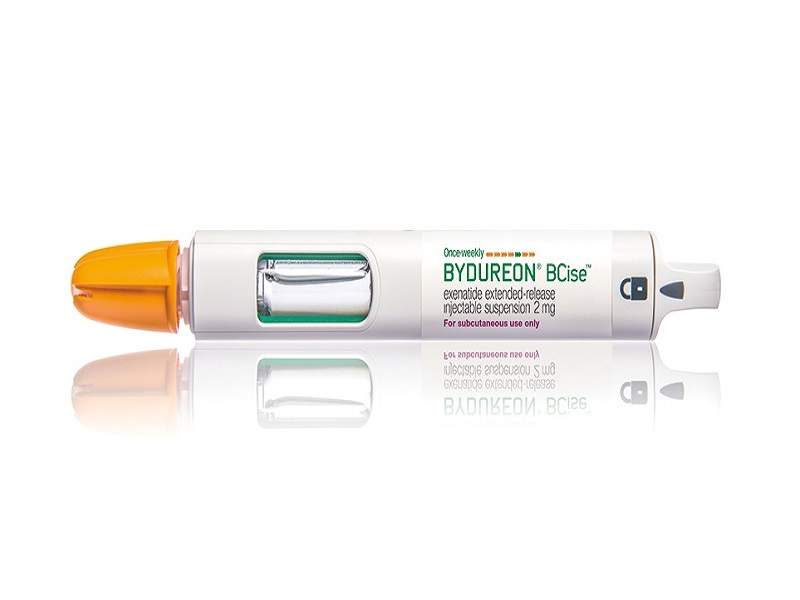
Bydureon® BCise™ (exenatide extended-release) is an injectable suspension containing a glucagon-like peptide-1 (GLP-1) receptor agonist.

Ozempic® (semaglutide) is a glucagon-like peptide (GLP-1) receptor agonist indicated for the treatment of Type 2 diabetes in adults.
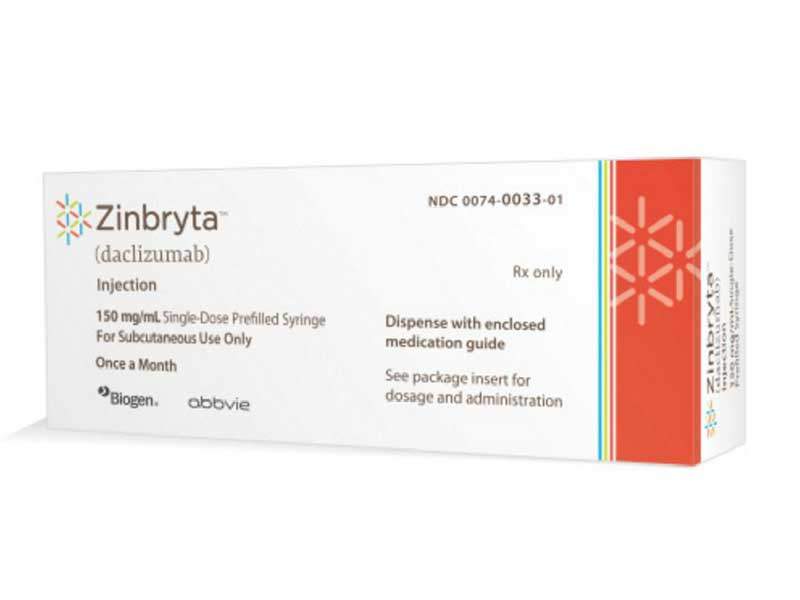
Zinbryta (daclizumab) is an injectable formulation jointly developed by Biogen and Abbive for the treatment of relapsing forms of multiple sclerosis (MS) in adults.
Vosevi™ (Sofosbuvir/Velpatasvir/Voxilaprevir) is a direct-acting antiviral (DAA) indicated for the treatment of chronic hepatitis C virus (HCV) infection in adults with genotypes one to six.
Haegarda® (C1 Esterase Inhibitor) a subcutaneous prophylactic therapy indicated for the prevention of hereditary angioedema (HAE) attacks in adolescent and adult patients.
Dinutuximab beta is an anti-GD2 monoclonal antibody indicated for the treatment of neuroblastoma in patients aged one year and above.
Ingrezza (valbenazine) is first and only US Food and Drug Administration (FDA) approved vesicular monoamine transporter 2 (VMAT2) inhibitor indicated for the treatment of adults with Tardive dyskinesia (TD).
Zejula™ (niraparib) is a poly adenosine diphosphate (ADP) ribose polymerase (PARP) inhibitor approved for the treatment of adult patients with ovarian cancer.
Emflaza™ (deflazacort) is the first glucocorticoid drug approved in the US for treatment of patients aged two years and older suffering from duchenne muscular dystrophy (DMD).