Alprolix [Coagulation Factor IX (Recombinant), Fc Fusion Protein] is an intravenous injection indicated for the control and prevention of bleeding episodes, as well as routine prophylaxis in haemophilia B-affected adults and children aged 12 and above. The drug was developed and marketed by Biogen idec in collaboration with Swedish Orphan Biovitrum (Sobi).
Health Canada approved Alprolix for the control and prevention of bleeding episodes and routine prophylaxis in haemophilia B patients in March 2014.
Biogen idec received the US Food and Drug Administration’s (FDA) approval for Alprolix in March 2014 for the control and prevention of bleeding episodes, perioperative (surgical) management and routine prophylaxis in adults and children with haemophilia B.
Alprolix also received approval from regulatory authorities in Australia (April 2014), Japan (July 2014) and New Zealand (September 2015).
The European Medicines Agency (EMA) approved Alprolix for the treatment of Haemophilia B in May 2016.
Haemophilia B: disease details
Haemophilia B is a rare hereditary blood clotting disorder caused by factor IX deficiency. Factor IX is one of the proteins needed for blood clotting.
It can lead to repeated bleeding episodes and may cause pain, irreversible joint damage and life-threatening haemorrhages.
The disease is estimated to affect approximately 3,300 people in the US. According to a global survey conducted by the World Federation of Hemophilia in 2011, 25,000 people were estimated to be diagnosed with haemophilia B worldwide.
Alprolix’s mechanism of action
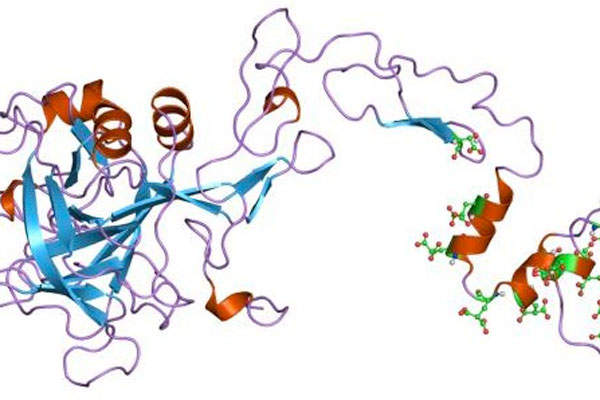
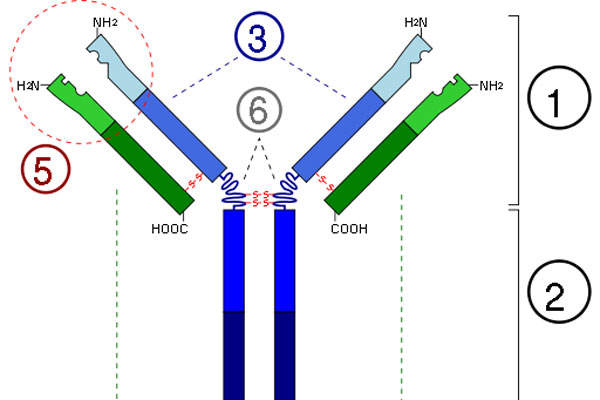
Alprolix is a recombinant clotting factor therapy prepared by combining factor IX protein to the Fc region of immunoglobulin G subclass 1 antibody. The drug replaces the missing coagulation Factor IX for a long period.
The drug is available in one single use vial with a 5ml prefilled diluents syringe for intravenous administration.
Clinical trials on Alprolix
Approvals for Alprolix were obtained from a global phase III clinical study known as B-LONG. It was an open-label, multi-centre clinical study that enrolled 123 males aged 12 and above with haemophilia B. It was conducted in 50 haemophilia treatment centres located across 17 countries in six continents.
The study evaluated the efficacy, safety and pharmacokinetics of Alprolix through four treatment regimens, including weekly prophylaxis arm, individualised-interval prophylaxis dosing arm starting at every ten days, episodic or on-demand therapy as needed to manage bleeding episodes, and perioperative management arm.
An intravenous drug indicated for the treatment of bleeding disorder in patients with factor XIII deficiency.
Results demonstrated that the adults and adolescents with severe haemophilia B who were given Alprolix once weekly or once every ten to 14 days had prevented or reduced the bleeding episodes with prophylactic infusions in a safe and effective manner.
The overall annualised bleeding rates (ABR) or projected rate of bleeding episodes in a year reported in weekly prophylaxis arm, individualised-interval prophylactic regimens arm and on-demand treatment arm included 2.95, 1.38 and 17.69 respectively. The average dosing interval in individualised-interval prophylaxis was 12.5 days, while it was 13.8 days in the last six months of the study.
Common adverse events found in the Alprolix 1, 2 and 3 groups during the clinical study included the common cold, flu, joint pain, upper respiratory tract infection, high blood pressure and headache.
Biogen idec initiated a phase III paediatrics study on Alprolix in February 2012. Also known as Kids B-LONG study, it was an open-label, non-randomised and parallel assignment enrolling 26 patients. Positive results were obtained from the study in February 2015.
The primary outcome measure of the study was the frequency of inhibitor development in 50 weeks. The secondary outcome measures included the number of annualised bleeding episodes and assessments of response to treatment with Recombinant factor IX-Fc fusion protein (rFIXFc) for bleeding episodes in 50 weeks.
Marketing commentary
Alprolix is the first approved long-acting haemophilia B therapy. Biogen Idec holds the rights to develop, manufacture and market Alprolix in North America.
Sobi holds the right to develop and commercialise Alprolix in Europe, Russia, the Middle East and Northern Africa.