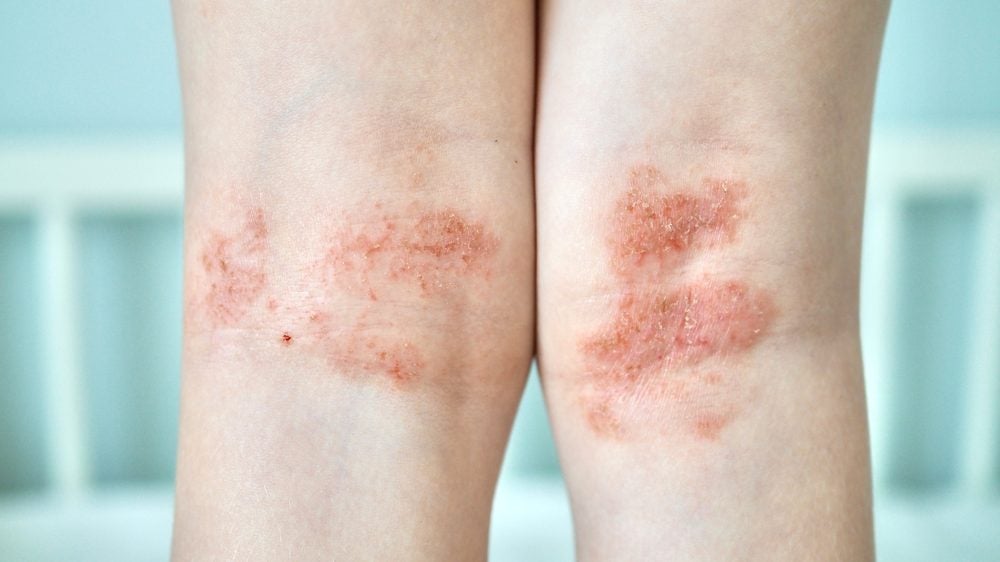
AbbVie has released long-term efficacy and safety data from three Phase III trials investigating Rinvoq (upadacitinib) in moderate-to-severe atopic dermatitis.
The 140-week data analysis from Measure Up 1 (NCT03569293), Measure Up 2 (NCT03607422) and AD Up (NCT03568318) showed that 15mg and 30mg Rinvoq-treated patients achieved significantly higher skin clearance compared to the placebo cohort.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Improvement in skin clearance was measured by the eczema area and severity index of 75 (EASI 75) and validated investigator global assessment for atopic dermatitis of 0 or 1 (vIGA-AD 0/1).
Across the three trials, patients treated with a 30mg dose of Rinvoq achieved the highest improvement in EASI 75 and vIGA-AD 0/1 at week 140. Participants treated with a 30mg dose in the Measure Up 2 trial achieved the biggest EASI 75 improvement (90.7%), whereas the highest vIGA-AD 0/1 improvement was observed in the 30mg cohort in the Measure Up 1 trial (65.5%).
Patients treated with Rinvoq also achieved better itch reduction (WP-NRS 0/1), which was measured as an additional endpoint compared to the placebo cohorts.
AbbVie’s vice-president of global medical affairs in immunology Dr Mudra Kapoor said: “Patients with moderate-to-severe atopic dermatitis often face relentless itch and inflammatory skin symptoms that can impact their everyday lives. These results reinforce our commitment to providing an effective, long-term treatment option for those living with this debilitating disease and other chronic, immune-mediated conditions.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataBoth 15mg and 30mg doses of Rinvoq were well tolerated and the safety profile was consistent with previous studies. The new data analysis demonstrated that long-term and continuous use of Rinvoq has an “acceptable benefit and risk profile” as a treatment for atopic dermatitis.
The three trials enrolled adult and adolescent (12 years or older) patients with moderate-to-severe atopic dermatitis. In the AD Up trial alone, Rinvoq was administered in combination with topical corticosteroids. In all trials, patients in the placebo cohort were switched to either the 15mg or 30mg dose of Rinvoq at week 16.
The US Food and Drug Administration approved Rinvoq as a treatment for moderate-to-severe atopic dermatitis in January 2022.
In July 2023, AbbVie started a Phase III trial (NCT05889182) with Rinvoq in hidradenitis suppurativa patients. The company plans to recruit 1,328 patients across 275 sites worldwide. According to the ClinicalTrials.gov registry, the estimated study completion date is August 2027.



