On May 3, Biogen announced plans to “substantially eliminate” its marketing budget for Aduhelm, effectively throwing in the towel on what was once anticipated to be a blockbuster drug for Alzheimer’s disease. Biogen said it was parting ways with CEO Michel Vounatsos the same day, and the company is now left looking back on what went wrong.
With the future of Aduhelm appearing bleak, we highlight three steps other biotechs can take to escape the same pitfalls of Aduhelm’s drug development flop: choose clinically meaningful endpoints wisely, transparently publish data as it becomes available, and don’t underestimate the effect of negative public opinion.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
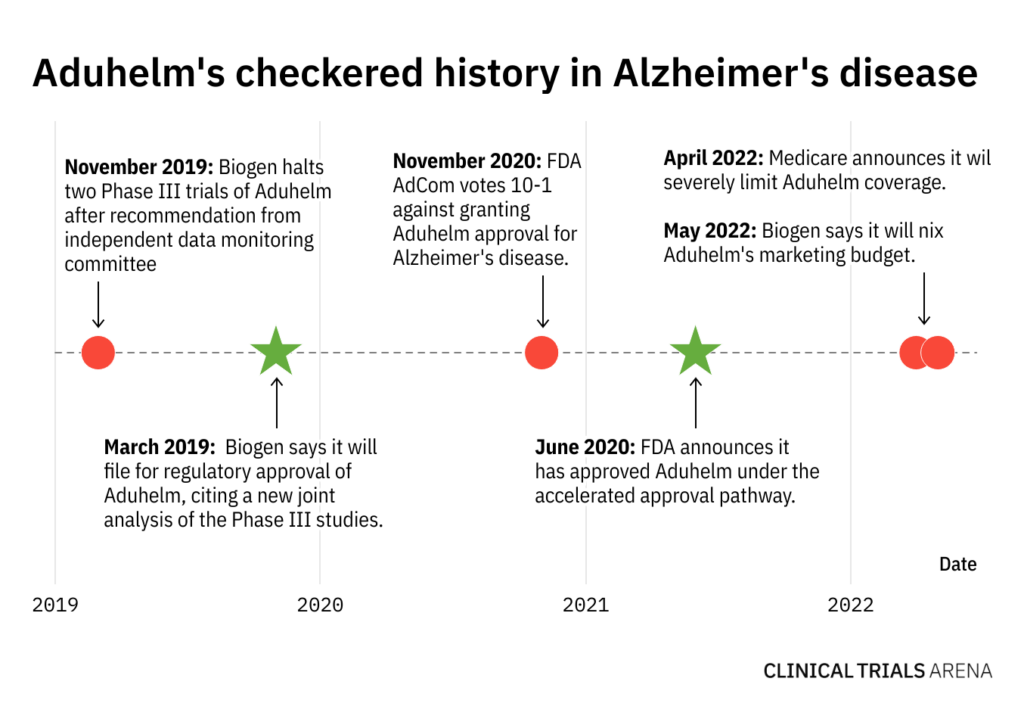
Aduhelm has had a rocky road to approval, marked by a series of setbacks and surprising regulatory decisions. In March 2019, Biogen announced it had halted two Phase III trials of Aduhelm after an independent data monitoring committee said the trials were unlikely to achieve their primary endpoints. But just seven months later, the biotech announced it would seek regulatory approval for the same drug.
In November 2020, an FDA Advisory Committee panel overwhelmingly voted against Aduhelm’s approval by a margin of 10-1, leading many analysts to consider the therapy all but dead in the water. Nevertheless, the FDA surprisingly granted the antibody accelerated approval in June 2021, skyrocketing Biogen’s stock price on the heels of bullish multi-billion dollar sales forecasts.
Since Aduhelm’s shocking FDA approval, Biogen has been hit by a myriad of devastating setbacks, culminating with Tuesday’s news that Vounatsos’ tenure as CEO was ending. Medicare announced it would severely restrict Aduhelm coverage, and many major healthcare providers and insurers have refused to pay for the treatment altogether. With dwindling uptake and grim sales forecasts, Aduhelm now stands as a crushing example of what can go wrong in drug development.
Choose Alzheimer’s disease endpoints wisely
Alzheimer’s disease is characterised by decades of gradual memory loss and brain atrophy, making the most common clinical outcome measures relatively insensitive over short periods. As such, a statistically significant slowing in disease progression is not enough for regulators and payers; clinical trials must prove the numbers on their primary endpoint scales translate to tangible effects for patients.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataWell before the FDA’s regulatory decision on Aduhelm, experts expressed concerns that the small, albeit statistically significant, change in the Clinical Dementia Rating Scale – Sum of Boxes (CDR-SB) lacked any clinically meaningful benefit. Similarly, a Phase II trial of Eli Lily’s donanemab in Alzheimer’s disease left experts skeptical that marginal changes in its primary endpoint of Integrated Alzheimer’s Disease Rating Scale (iADRS) actually benefited patients.
Publish clinical trial data transparently
When Medicare announced it would severely restrict coverage of Aduhelm except in the case of clinical trials, the agency cited concerns with the drug’s safety. Chief among them was ARIA, a type of brain swelling and bleeding that often resolves naturally but is not fully understood.
But Biogen failed to publicly share detailed safety data on ARIA from clinical trials in a timely and transparent manner, drawing pause and ire among many prominent clinicians. When a biotech lacks transparent data-sharing practices, regulators and payers could end up assuming the worst.
Don’t underestimate public opinion
When the FDA approved Aduhelm, Biogen’s stock skyrocketed to record highs percent behind bullish analyst reports forecasting billions in sales. But then multiple AdCom members resigned in protest and widespread public backlash prompted prominent institutions like the Cleveland Clinic to block Aduhelm’s use.
While its crucial to gain support from regulators, that doesn’t guarantee support from clinicians and the broader public. Drug manufacturers must convince clinician and patient communities that their treatments are effective outside of the clinical trial setting, heeding the public outcry against Aduhelm as a warning of what could go wrong.





