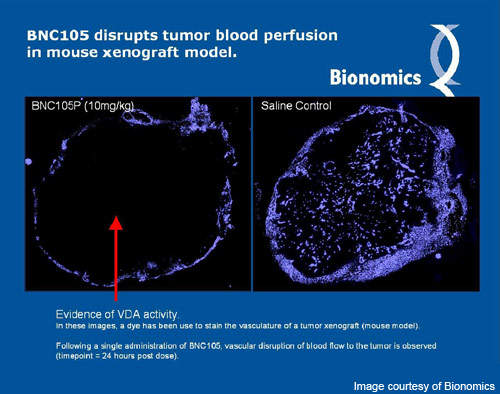
BNC105, being developed by Adelaide-based Bionomics, is a new kind of drug known as a vascular targeting agent (VDA). As a VDA, the drug is targeted at treating different types of cancer. BNC105 has shown positive results in Phase I clinical trials conducted in patients with advanced cancers.
The drug is in Phase II of development and is being tested in patients with renal cell carcinoma and mesothelioma.
In August 2012, Bionomics was granted a patent for BNC105 by the US Patent and Trademark Office for a period of 20 years till February 2027. Bionomics already holds patents for BNC105 in Australia and New Zealand.
Cancer and the need for new treatment forms
Cancer, also known as malignancy, occurs when there is abnormal or uncontrolled growth of cells. Such growth often leads to destruction of adjacent tissues and can also result in metastasis or spreading to other areas of body via blood or lymph.
The additional cells created sometimes take the form of a tumour which can be benign or malignant. Benign tumours can be taken out and rarely develop again. Malignant tumours, however, can spread to other parts of the body and affect other organs.
There are over 100 different types of cancer, each of them normally named after the organ or type of cell which is affected. Cancer is broadly categorised as carcinoma (cancer of the skin), sarcoma (cancer of the bone, muscle or other supportive tissue), leukaemia (cancer of bone marrow), lymphoma, myeloma (cancer of the immune system) and central nervous system cancers (cancers that begin in the tissues of the brain and spinal cord).
The most commonly occurring cancer is non-melanoma skin cancer. In 2009, more than 1,000,000 cases of this cancer were recorded in the US. Other cancers that are the leading causes of death in the US include bladder, breast, colon and rectal cancers.
On diagnosis, a combination of treatments is used to tackle cancer. These include chemotherapy, radiation and surgery. New treatments that specifically target the tumour-causing cells and minimise the damage caused to normal cells are evolving, however.
BNC105 – vascular targeting agent
BNC105 was developed using Bionomics’ proprietary MultiCore technology used to create novel compounds.
The mechanism of action of BNC105 is similar to that of any VDA. VDAs act by cutting the blood supply to tumours, thereby depleting them of oxygen and nutrients required for survival. Such an approach to treating cancer is much more effective than other conventional treatments, as killing a single blood vessel can eliminate thousands of tumour cells.
Compared to other VDAs BNC105 has a higher therapeutic index, which means that a bigger window exists between one effective dose and a dose at which toxic effects are observed. Being more selective for blood vessels of tumours renders the enhanced therapeutic index to BNC105.
In addition to being a VDA, BNC105 is a cytotoxic agent or tubulin polymerisation inhibitor. BNC105 targets only the cancer cells and acts as a toxic agent to only those cells and not the normal cells. It can be combined with other treatments such as radiation therapy to eliminate tumours.
Another unique feature of BNC105 is that it is cleared from normal cells quickly and is retained within the tumours. This “lock-in” effect has provided better treatment response to the drug and also showed fewer side-effects in clinical trials.
Clinical trials demonstrate BNC105’s effect on tumours
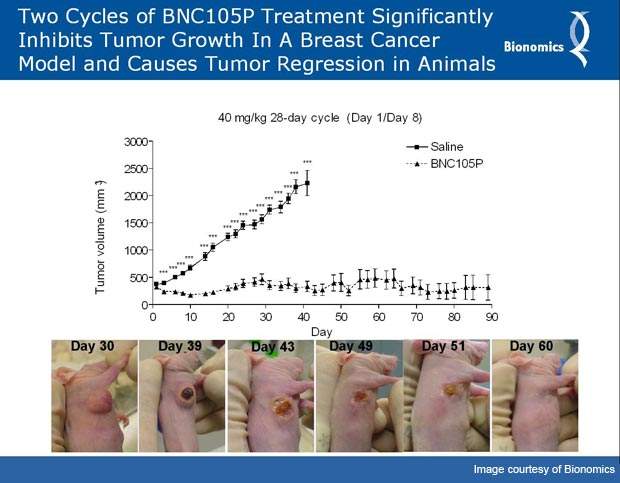
In preclinical studies, BNC105 showed its ability to inhibit tumour growth. In January 2008, a Phase I clinical trial was initiated under an Investigational New Drug Application approved by the US Food and Drug Administration. Bionomics carried out the trial at the Peter MacCallum Cancer Centre, the Western Hospital, Austin Health and the Royal Melbourne Hospital. The trial showed positive results in patients suffering from advanced cancers.
Bionomics initiated a Phase I clinical trial on BNC105 to compare its safety and tolerability in combination with Novartis’ everolimus (afinitor). The study is being conducted on 12 patients. Five patients have completed over 10 cycles of treatment and two more patients are still undergoing the treatment. The preliminary results of the study have indicated that the combination of BN105 with Afinitor was well tolerated.
In June 2009, Bionomics initiated Phase II clinical trials for BNC105 to test the safety and efficacy of the drug in combination with afinitor in patients suffering from renal cell carcinoma (RCC). Bionomics contracted Hoosier Oncology Group to carry out the trial in 12 centres across the US.
In December 2009, a second Phase II trial known as Mesothelioma trial was initiated by Bionomics to test the safety and efficacy of the drug in 30 mesothelioma patients. The study enrolled patients who received first line chemotherapy with pemetrexed (Alimta) and cisplatin.
The company engaged Australasian Lung Cancer Trials Group and the NHMRC Clinical Trials Centre to carry out the trial. Preliminary results have indicated that a 16mg/m² dose of BNC105 was well tolerated.
BNC105 is also being evaluated in a phase I/II clinical trial for ovarian cancer in combination with carboplatin and gemcitabine. The trial is being carried out across sites in Australia, New Zealand and the US.
Marketing commentary
BNC105 has significant potential for the treatment of different types of cancers. The potential market for VDAs is estimated at about $5bn annually. Although Bionomics will not market BNC105, it is expected to seek interest from pharmaceutical companies to carry out the next stage of development of the drug.