Elelyso (taliglucerase alfa) is an intravenous drug indicated for the treatment of type one Gaucher disease. The drug was jointly developed by Protalix BioTherapeutics and Pfizer.
Protalix and Pfizer received the US Food and Drug Administration’s (FDA) approval for Elelyso for the treatment of adult patients with type one Gaucher disease, in May 2012. Protalix received marketing authorisation for Elelyso in Israel in September 2012.
The marketing authorisation for Elelyso in Europe was rejected by the European Commission (EC) in October 2012. Elelyso’s European marketing authorisation was rejected because Vipriv (velaglucerase alfa), a drug produced by Shire for the same indication, had already received exclusive marketing authorisation for ten years under orphan drug designation.
Gaucher disease causes and symptoms
Gaucher is a genetic disorder which occurs due to the deficiency of glucocerebrosidase enzyme production. The deficiency of glucocerebrosidase enzyme leads to formation of lipids in spleen, liver, kidneys and other organs in the body.
Type one of Gaucher disease is the mild and most common form of the disease affecting about 90% of the patients. The symptoms of the disease include anaemia, fatigue, easy bruising and a tendency to bleed. The disease could lead to spleen damage, bone problems and anaemia.
Type 1 Gaucher disease is estimated to affect about 6,000 people in the US. It was considered an orphan disease by the FDA.
Mechanism of action of Elelyso
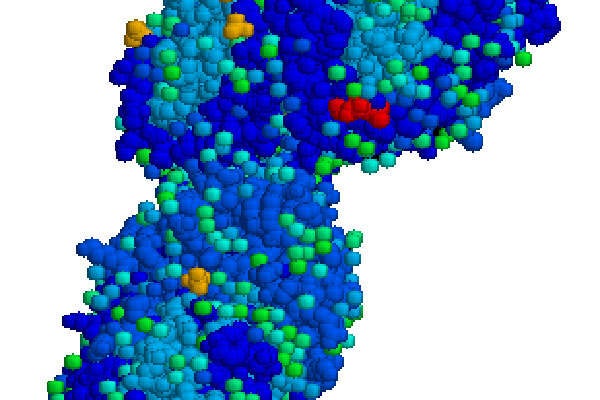
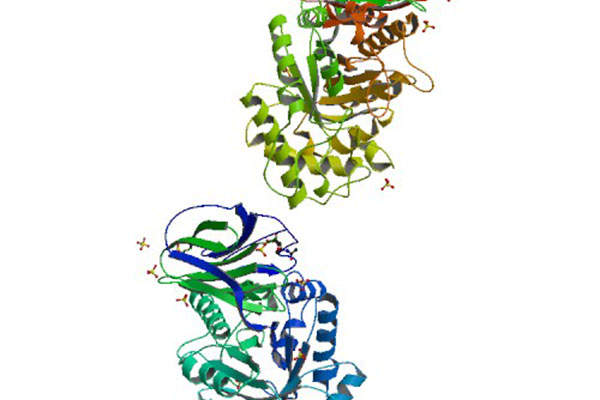
Elelyso contains a recombinant glucocerebrosidase enzyme, known as taliglucerase alfa. The drug works by catalysing the hydrolysis of non-functional glucocerebroside to glucose and lipid molecules. The drug is administered intravenously through an injection.
Clinical trials on taliglucerase alfa
FDA approval for Elelyso was based on the results from two Phase III clinical studies conducted for nine months.
The first Phase III clinical trial was a randomised, double blind and multicentre clinical study. It was conducted between August 2007 and October 2009 in numerous study centres located across US, Canada, UK, Spain, Italy, Israel, South Africa and Chile. The study enlisted 31 adult patients with Gaucher disease. The patients were treated with Elelyso doses of either 30 units/kg or 60 units/kg.
The primary endpoint of the study was finding the change in spleen volumes (SD) after nine months, through MRI.
The results of the study demonstrated that the patients treated with 30 units/kg dose showed a mean change of -4.5 in spleen volumes (SD), whereas the patients treated with 60 units/kg dose of Elelyso showed a change of -6.6 in spleen volumes. The study also observed changes in liver volumes and haemoglobin concentrations.
The second Phase III clinical study was an open label, single arm study. It was conducted between December 2008 and March 2013. It enrolled 25 patients with type 1 Gaucher disease. The patients for the study were those who were previously administered with biweekly dose of imiglucerase for at least six months. The patients were administered with Elelyso in similar doses to that of imiglucerase.
The study’s results showed that the organ volumes and haematologic values remained stable on average in the nine months of treatment period. The side effects associated with Elelyso, observed during the clinical studies, included infusion reactions, nasopharyngitis, throat infection, headache, arthralgia, influenza / flu, UTI / pyelonephritis, back pain and extremity pain.
Marketing commentary for Pfizer / Protalix’s drug
Protalix BioTherapeutics and Pfizer entered into an agreement for the joint worldwide development and commercialisation of Elelyso, in November 2009. The agreement allows Protalix to retain the commentarial rights of Elelyso in Israel, while Pfizer was licensed to develop and commercialise Elelyso worldwide.
Pfizer agreed upon a milestone payment of $8.3m to Protalix under a Clinical Development Agreement signed between the two companies in December 2012. Protalix will take care of the ongoing clinical trials on the drug, including sponsoring of the trials, while Pfizer will sponsor any new clinical trials on the drug, as part of the agreement.
Other medications available in the market for treatment of Gaucher disease include miglustat (Zavesca), which was developed by Actelion, and Cerezyme (imiglucerase), developed by Genzyme.
Related content
Signifor (Pasireotide) – Treatment for Cushing’s Disease, Germany
Signifor (SOM230 / Pasireotide) is a somatostatin analogue indicated for the treatment of patients with Cushing’s disease for whom surgery has failed.
Semagacestat – Gamma Secretase Inhibitor for Alzheimer’s Disease
Semagacestat (LY-450139) was a gamma secretase inhibitor being developed as a treatment for Alzheimer’s disease by Eli Lilly.