Epkinly™ (epcoritamab-bysp) is a T-cell engaging bispecific antibody indicated for the treatment of adults with relapsed or refractory (r/r) diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS), including DLBCL originating from indolent lymphoma and high-grade B–cell lymphoma, who have received at least two or more lines of systemic therapy.
The drug is being jointly developed and commercialised by Genmab, a biotechnology company based in Denmark and AbbVie, a biopharmaceutical company based in the US, as part of a broad oncology agreement signed between them in June 2020.
The agreement included the co-development and marketing of three of Genmab’s investigational bispecific antibody product candidates, including epcoritamab, in the early stages of development. Additionally, the agreement included the initiation of a research collaboration between them focused on discovering innovative antibody therapeutics for cancer treatment in the future.
Epkinly is available as a clear to slightly opalescent, colourless to slightly yellow solution in single-dose vials in 4mg/0.8ml and 48mg/0.8ml dosage strengths for subcutaneous injection.
Regulatory approval for Epkinly
Genmab submitted a biologics license application to the US Food and Drug Administration (FDA) for epcoritamab in October 2022. The European Medicines Agency (EMA) too validated a marketing authorisation application for epcoritamab submitted to them by AbbVie.
Subsequently, Genmab submitted a Japanese new drug application to the Ministry of Health, Labour and Welfare of Japan for epcoritamab in December 2022.
Epkinly received accelerated approval for the condition based on the response rate and durability of response demonstrated in the Phase 1/2 EPCORE NHL-1 clinical trial from the FDA in May 2023.
The Committee for Medicinal Products for Human Use of the EMA endorsed a favourable viewpoint, suggesting the approval of conditional marketing authorisation for epcoritamab in July 2023. The drug will be marketed under the brand name Tepkinly® in Europe as a monotherapy for adult patients with r/r DLBCL following two or more rounds of systemic therapy if approved.
The final decision by the European Commission regarding this particular indication for epcoritamab is expected to be made later in 2023.
DLBCL causes and symptoms
DLBCL is a fast-growing blood cancer and the most common form of non-Hodgkin lymphoma (NHL) that develops in the lymphatic system when B-cells mutate, accounting for approximately 30% of all NHL cases.
The disease can arise in lymph nodes and organs outside the lymphatic system. Most commonly, it occurs in older people and is slightly more prevalent in men.
The most common causes of the disease include certain viruses, including Epstein-Barr virus, human immunodeficiency virus (HIV), hepatitis B and hepatitis C, immunosuppressant drugs taken after organ transplants, autoimmune disorders, immunodeficiency, increased body mass index, agricultural pesticides and ionising radiation.
The symptoms primarily include swollen lymph nodes, generally noticed in the neck, armpit, or groin.
Epkinly’s mechanism of action
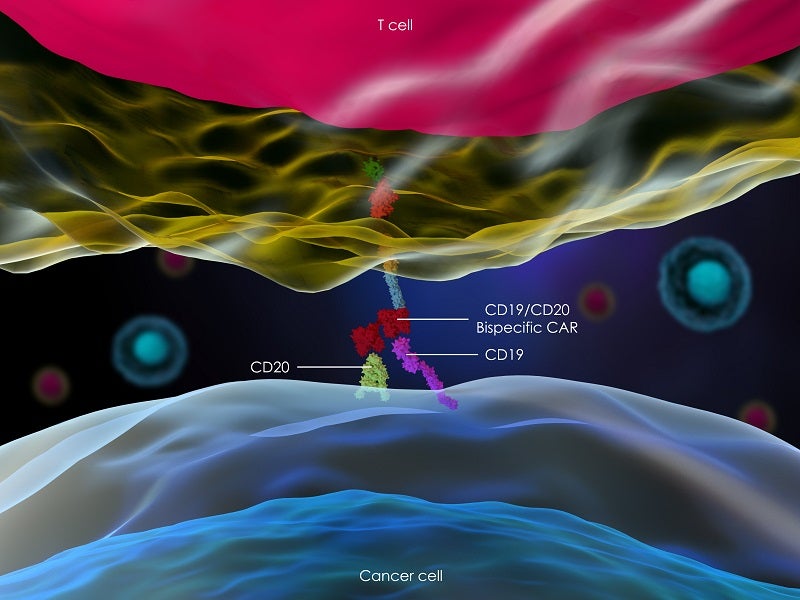
Epkinly is a bispecific CD20-directed CD3 T-cell engager created utilising Genmab’s DuoBody® technology platform. The technology is designed to direct cytotoxic T-cells in a targeted manner to induce an immune reaction against specific types of target cells.
The drug employs a dual-targeted strategy for simultaneous binding with both the CD3 receptor expressed on the surface of T-cells and CD20 expressed on the surface of lymphoma cells and healthy B-lineage cells.
Clinical trials on Epkinly
Epkinly was evaluated in an open-label, multi-cohort, multi-centre, single-arm clinical trial named EPCORE NHL-1.
The study consists of three parts, namely, the Phase 1 first-in-human, dose-escalation part; the Phase 2a expansion part and the Phase 2a optimisation part. The data from the expansion cohort of the trial in 157 patients with r/r LBCL formed the basis of its approval by the FDA.
Among 157 enrolled patients, 148 patients had DLBCL or high-grade B-cell lymphoma, of which 89% were diagnosed with DLBCL NOS.
The study aimed to evaluate the safety and efficacy of epcoritamab in the participants receiving epcoritamab at the recommended Phase 2 dose in three cohorts of patients with different types of r/r B-cell NHL, who have limited therapeutic options.
Epkinly showed an overall response rate of 61% with 38% complete response. The median duration of response was 15.6 months in the patients.
The most common adverse reactions during the clinical trials were cytokine release syndrome, fatigue, musculoskeletal pain, injection site reactions, pyrexia, abdominal pain, nausea and diarrhoea.