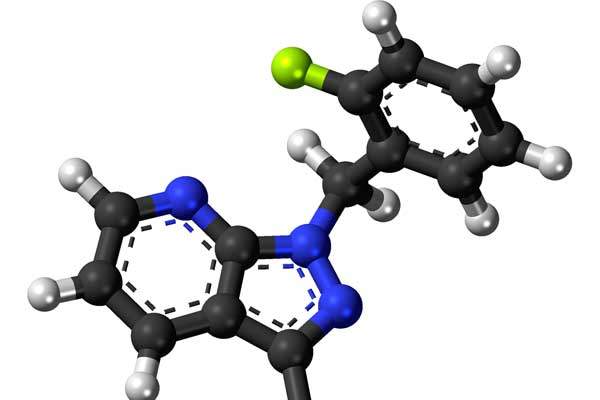
Keveyis (dichlorphenamide) is the first medicine indicated for the treatment of primary hyperkalemic and hypokalemic periodic paralysis, which are a group of rare hereditary disorders that cause occasional episodes of muscle weakness or paralysis.
Keveyis 50mg tablet was approved by US Food and Drug Administration (FDA) for the treatment for primary hyperkalemic and hypokalemic periodic paralysis and related variants in August 2015.
Keveyis was discovered and developed by Taro Pharmaceutical Industries.
Diplomat Pharmacy, a speciality pharmacy based in Michigan, will be the only authorised distributor of the drug in the US. The drug will be made available in the US in the third quarter of 2015.
Periodic paralysis and its variants
Periodic paralysis (PP) is a heterogeneous group of hereditary muscle disorders characterised by episodes of muscle weakness or paralysis occurring at irregular intervals. They are broadly classified into primary and secondary disorders. Common types of primary PP include hyperkalemic and hypokalemic.
The US is estimated to have approximately 5,000 people affected with primary PP. Common characteristics of the disorder include genetic mutations, alteration of potassium levels in blood and defective ion channels.
Keveyis mechanism of action
Keveyis contains an active ingredient called dichlorphenamide, which is a carbonic anhydrase inhibitor.
Carbonic anhydrase inhibitors partially suppress the secretion of aqueous humour through which they reduce the intraocular pressure. The complete mechanism of action of the drug in exerting its therapeutic effect is unknown.
Keveyis is available in the form of 50mg tablets for oral administration only.
Clinical trials on Keveyis
Ruconest was approved by US Food Drug and Administration in July 2014 for treatment of acute angioedema attacks in adult and adolescent patients with HAE.
The approval of Keveyis by the FDA was based on results from two clinical trials, namely Study 1 and Study 2, conducted to evaluate the efficacy of the drug in patients with hyperkalemic and hypokalemic periodic paralysis.
The primary endpoint for both studies was the average number of muscle weaknesses recorded per week over the trial period of eight weeks. Another endpoint considered was withdrawal from the study due to acute severe worsening.
Study 1 was a nine-week, double blind, placebo-controlled, multi-centre study comprised of two sub-studies. Sub-study 1 was conducted on patients with hypokalemic periodic paralysis and the other on patients with hyperkalemic periodic paralysis.
Treatment-naive subjects received 50mg of Keveyis, while those already on dichlorphenamide continued as normal. Those who were taking acetazolamide prior to the study were given Keveyis at 20% of the acetazolamide dose. Sub-study 1 enrolled subjects of a median age of 45 years, of which 73% were male.
Results demonstrated the subjects treated with Keveyis had 2.2 fewer attacks a week compared to those on a placebo, while none reached the endpoint of acute worsening, compared with five patients on a placebo.
43% of sub-study 2 were male and of a median age of 43 years. Subjects treated with Keveyis during the study had 3.6 fewer attacks compared to those treated with a placebo, while none reached the endpoint of acute worsening, compared with two using aplacebo.
Study 2 was a 35-week, multi-centre, double-blind, placebo-controlled, two-period crossover study that consisted of two sub-studies.
One sub-study was on patients with hypokalemic PP and the other was in those with hyperkalemic PP, including patients with paramyotonia congenita, a disorder that affects muscles used for movement.
The primary endpoint in sub-study 1 was the incidence of acute intolerable worsening necessitating withdrawal, while in the second sub-study it was the average number of self-reported attacks of muscle weakness per week.
Sub-study 1 recruited patients with a mean age of 38 years that constituted 79% of men. The primary endpoint was observed in two patients on Keveyis, compared with 11 patients on a placebo.
Sub-study 2 was conducted on patients that had a mean age of 37 years and constituted of 79% of men. The subjects treated with Keveyis had 2.3 fewer attacks a week than those on a placebo.
The most common adverse reactions recorded during the study were paresthesias, cognitive disorder, dysgeusia (distortion of the sense of taste), and confusion.