Mekinist (trametinib) is a mitogen-activated protein kinase (MEK) inhibitor, indicated for the treatment of unresectable or metastatic melanoma in adult patients. The drug is developed and marketed by GlaxoSmithKline (GSK).
GSK submitted a New Drug Application (NDA) for Mekinist to the US Food and Drug Administration (FDA), for the treatment for patients with unresectable or metastatic melanoma, in August 2012. The FDA approved Mekinist for the treatment of metastatic melanoma with BRAF V600E or V600K mutations in adult patients in May 2013.
GSK submitted a marketing authorisation application for Mekinist to the European Medicines Agency (EMA) in February 2013. The EMA granted marketing authorisation for the drug as a single agent to treat adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation, in July 2014.
GSK retains all rights to develop, manufacture and commercialise the drug worldwide expect in Japan where Japan Tobacco holds the co-promotion rights of the drug.
Metastatic melanoma causes and types
Melanoma is a fast growing cancer that originates in melanocytes, which are responsible for making the skin colour. It is the most dangerous and fatal form of skin cancer that initially emanates as a single tumour and spreads to the entire body.
When the disease spreads to other parts of the body, it is known as advanced or metastatic melanoma.
Melanoma will result in about 9,480 deaths across the US in 2013, according to the National Cancer Institute’s estimates. It is also found that about half of the metastatic melanoma patients have a BRAF gene mutation, which enables melanoma tumours to grow and spread in the body.
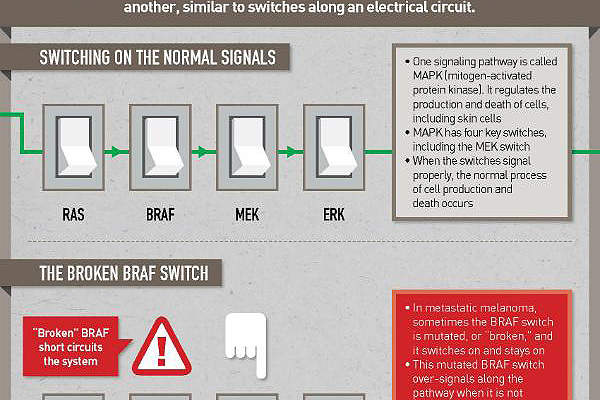
Mekinist’s (trametinib) mechanism of action
Mekinist contains trametinib, which comprises of an MEK inhibitor drug. The drug stops the MEK1 and MEK2 kinase activity which promotes the cellular proliferation. It also activates BRAF pathway and inhibits the melanoma cell growth. The drug is available in 0.5mg and 2mg dosed tablets for oral administration.
Clinical trials on GSK’s Mekinist (trametinib)
The FDA and EMA approvals for Mekinist were based on the results obtained from an open label Phase III clinic trial known as METRIC study. The study was conducted between November 2010 and March 2013, in 112 locations across the world. It was a randomised and international study which enrolled 322 metastatic melanoma adult patients with BRAF V600E or V600K mutation.
The patients selected were above 18 years of age and previously treated with no more than one chemotherapy regimen.
Patients were administered with Mekinist or chemotherapy in a 2:1 ratio, respectively. The primary outcome measure of the study was progression-free survival (PFS). The secondary outcome measures included PFS and overall survival (OS) in all patients, and the duration of response.
The results of the study demonstrated that patients treated with Mekinist showed statistically significant increase in PFS when compared to the chemotherapy group. The median PFS in Mekinist administered patients was 4.8 months, while it was 1.5 months in the chemotherapy group. The study met the primary and secondary endpoints.
The common adverse reactions found in Mekinist administered patients during the clinical study included rashes, diarrhoea, lymphoedema, dermatitis acneiform, stomatitis, hypertension, abdominal pain, haemorrhage, paronychia, pruritis and dry skin.
Marketing commentary for GlaxoSmithKline’s drug
The MEK protein inhibitor was discovered by Japan Tobacco and in-licensed by GSK in 2006.
Japan Tobacco retains the co-promotion rights of Mekinist in Japan.
The drug was in-licensed by GSK in 2006.
GSK entered into an agreement with bioMérieux to co-develop Mekinist, in 2010. bioMérieux provided assistance in the clinical diagnostic tests to detect BRAF V600 gene mutations with its FDA-approved THxID™-BRAF tests.
Other medications approved for the treatment of metastatic melanoma include Yervoy (Ipilimumab), developed by Bristol-Myers Squibb, and Zelboraf (vemurafenib) which is manufactured by Plexxikon and Roche.
Related content
Tafinlar (Dabrafenib) – Treatment for Metastatic Melanoma, US
Tafinlar (dabrafenib) is a single-agent oral treatment indicated for the treatment of unresectable or metastatic melanoma in adult patients.
Yervoy (Ipilimumab) – Therapy for Treatment of Metastatic Melanoma, US
Yervoy is a monoclonal antibody drug indicated for treating metastatic melanoma. The drug was developed by Bristol-Myers Squibb.