Olysio (simeprevir) is an antiviral drug indicated for the treatment of chronic hepatitis C (CHC) infection. The drug was jointly developed by Janssen Therapeutics and Medivir.
Janssen received approval for Olysio from the US Food and Drug Administration (FDA), in November 2013, for the treatment of hepatitis C genotype 1 infected adult patients.
Janssen also received positive opinion for the marketing authorisation application (MAA) for Olysio for the treatment of CHC infection from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) in March 2014.
The drug was approved for CHC in Japan in September 2013 and is being traded under the name Sovriad. It was also approved in Canada in November 2013 and is being sold under the brand name Galexos.
Hepatitis C – causes and severity
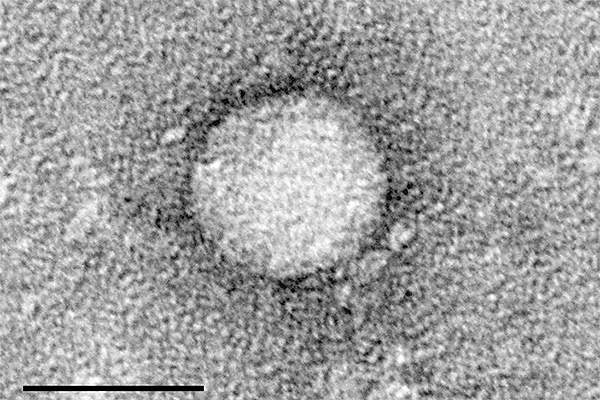
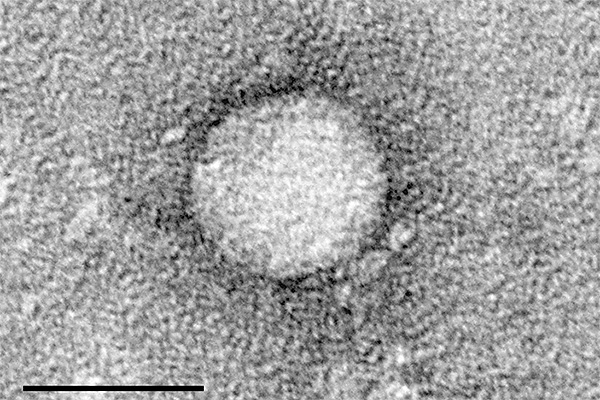
Hepatitis C is an infectious disease of the liver caused by hepatitis C virus (HCV). It may increase the risk of developing complications from cirrhosis, which may include liver failure.
The infectious disease is estimated to affect about 150million people across the world, of which about 350,000 people die from the disease every year. The disease is estimated to affect about 3.2million people in the US.
Olysio’s mechanism of action
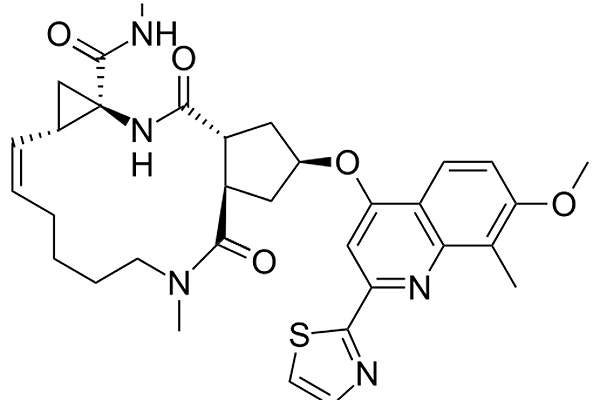
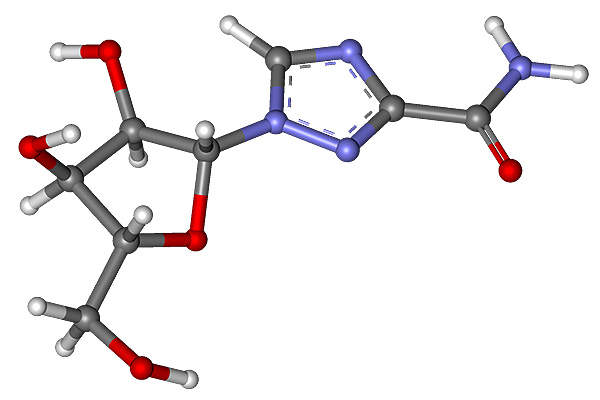
Olysio contains an active substance called simeprevir, a NS3/4A protease inhibitor. The drug is made in combination with peginterferon-alfa and ribavirin. The drug prevents viral maturation through inhibition of protein synthesis.
Olysio is available in capsule form in 150mg dose for oral administration.
Clinical trials on Olysio (simeprevir)
Janssen Submitted new drug application (NDA) for Olysio based on the results obtained from two Phase III clinical trials known as Quest 1 and Quest 2, and a Phase 2b study called ASPIRE. The clinical studies evaluated Olysio dosed once daily in combination with pegylated interferon and ribavirin, versus treatment with placebo plus pegylated interferon and ribavirin.
The results from the Quest 1 and Quest 2 studies demonstrated that 58% of the genotype 1a treatment-naïve patients with Q80K polymorphism who were administered with Olysio achieved sustained virologic response in 12 weeks (SVR12) after the end of treatment, compared to 84% of patients without the Q80K polymorphism. The patients who were administered with placebo achieved 52% SVR12 with the Q80K polymorphism.
The results from Quest 1 and Quest 2 pooled analysis showed that 80% of the Olysio treatment-naïve group patients achieved SVR12 after the end of treatment, compared to 50% of the patients who achieved it in the placebo groups.
The results from PROMISE study conducted on prior-relapser patients showed that the patients with the Q80K polymorphism who received Olysio achieved 47% SVR12 in comparison to 78% of patients who achieved it without the polymorphism. In the placebo arm 30% of the patients with the Q80K polymorphism achieved SVR12.
The results from ASPIRE study demonstrated that at the end of treatment, after 24 weeks, 65% of the prior partial-responder patients in Olysio group achieved SVR24 compared to nine percent of the patients in placebo group, and 53% of prior-null responder patients in Olysio group achieved SVR24, compared to 19% of patients in the placebo group.
Janssen initiated two more Phase III clinical trials on Olysio in April 2014. The clinical studies will examine the efficacy and safety of simeprevir in combination with the nucleotide inhibitor sofosbuvir.
The first trial, known as OPTIMIST-1 (optimal treatment with a simeprevir and sofosbuvir therapy), will be an open-label, randomised, multicentre study that will investigate the efficacy and safety of simeprevir 150mg in combination with sofosbuvir 400mg. The patients will be administered with the combination once daily for eight or 12 weeks. The combination will be used on HCV genotype 1 infected patients with cirrhosis who are HCV treatment naïve or treatment experienced.
The second Phase III clinical trial, known as OPTIMIST-2, will be an open-label, single-arm study that will investigate the efficacy and safety of simeprevir 150mg in combination with sofosbuvir 400mg. The combination will be used once daily for 12 weeks on HCV genotype 1 infected patients with cirrhosis who are HCV treatment naïve or treatment experienced.
The primary efficacy endpoint in both the OPTIMIST trials will be the proportion of patients achieving sustained virologic response 12 weeks after the end of treatment (SVR12). Both the studies will enrol about 400 patients in the US and Canada. Ribavirin will not be used in both the OPTIMIST trials.
Marketing commentary
Janssen Therapeutics holds the worldwide clinical development and marketing rights of Olysio excluding Nordic countries. Medivir retains the marketing rights for Olysio under the marketing authorisation held by Janssen-Cilag International in Nordic countries.
Other medications available in the market for the treatment of the same indication include Sovaldi (sofosbuvir) developed by Gilead Sciences, and Incivek (telaprevir) manufactured by Vertex Pharmaceuticals, Mitsubishi Tanabe Pharma Corporation and Tibotec BVBA.