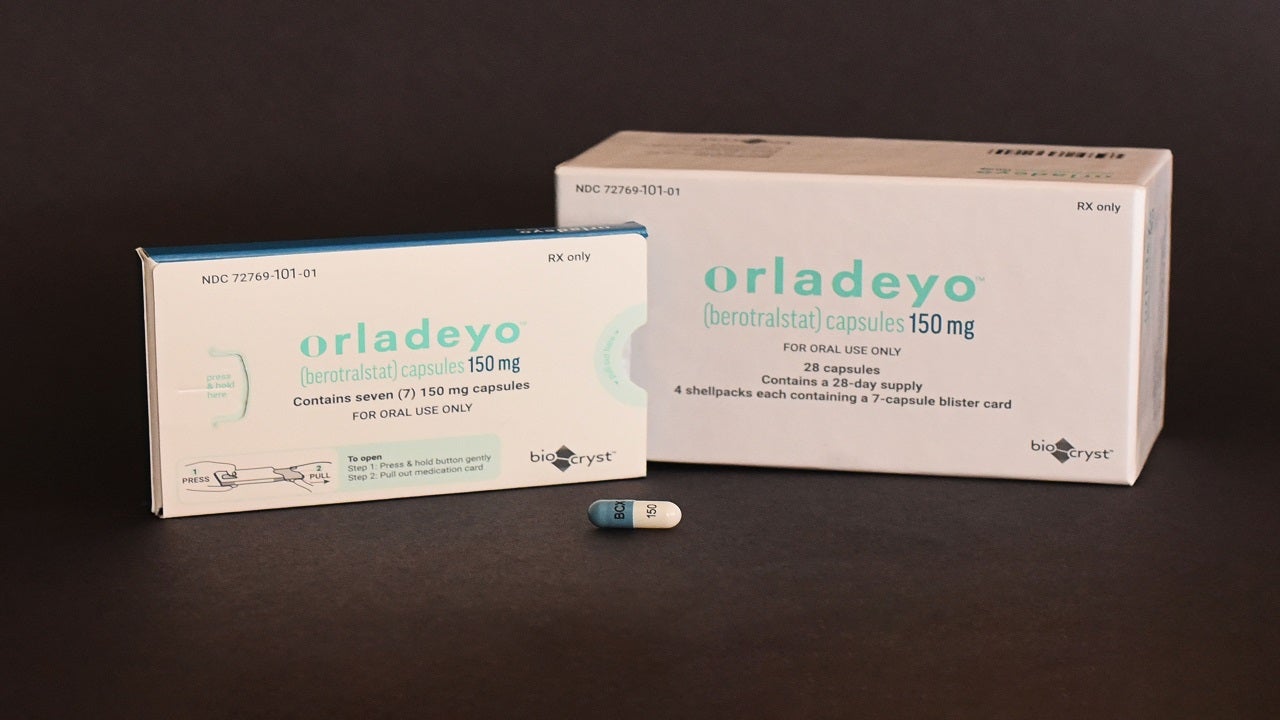
ORLADEYO™ (berotralstat) is a targeted oral prophylactic therapy indicated for the prevention of swelling attacks associated with hereditary angioedema (HAE) in adults and paediatric patients aged 12 years and older. It was developed by BioCryst Pharmaceuticals, a pharmaceutical company based in the US.
The drug is available as a hard gelatine oral capsule with a white opaque body for 150mg dosage strength, as well as light blue opaque body for 110mg dosage strength.
Patients and healthcare professionals receive ORLADEYO through a single point of contact via EMPOWER Patient Services, which receive the drug through Optime Care, an exclusive speciality pharmacy provider for the drug.
Regulatory approvals of ORLADEYO
ORLADEYO (BCX7353) received fast track designation from the US Food and Drug Administration (FDA) in August 2018. In November 2019, BioCryst partnered with Torii Pharmaceutical for the commercialisation of BCX7353 in Japan.
In December 2019, BioCryst submitted a new drug application (NDA) for the drug to the FDA, which was subsequently approved in December 2020. The drug gained orphan drug status in the European Union (EU) in June 2018.
In January 2021, ORLADEYO received marketing and manufacturing approval in Japan, where it also holds orphan drug and Sakigake designations.
In February 2021, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended the drug for approval in Europe. The drug was approved in Europe in April 2021. The approval covers all EU member states, as well as Iceland, Norway and Liechtenstein.
In May 2021, the United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA) approved the drug, following the submission of a marketing authorisation application by the company in March 2021.
In June 2021, the Israeli Ministry of Health accepted the regulatory submission of ORLADEYO for accelerated review. BioCryst has entered a distribution and supply agreement with Neopharm, an Israeli pharmaceutical company, to commercialise the drug in Israel.
In September 2021, the United Arab Emirates’ (UAE) Ministry of Health and Prevention (MOHAP) granted approval to ORLADEYO. BioCryst selected UAE-based pharmaceutical company NewBridge Pharmaceuticals for the regional distribution of the drug covering the Gulf Cooperation Council (GCC) and Iraq.
The drug was recommended by the UK’s National Institute for Health and Care Excellence (NICE) in September 2021.
In June 2022, Health Canada approved ORLADEYO. In the same month, BioCryst collaborated with Pint Pharma, an Austrian pharmaceutical company, to promote the drug in the pan-Latin America (LATAM) region. Pint Pharma will obtain all marketing authorisations for the drug and commercialise it in the pan-LATAM region.
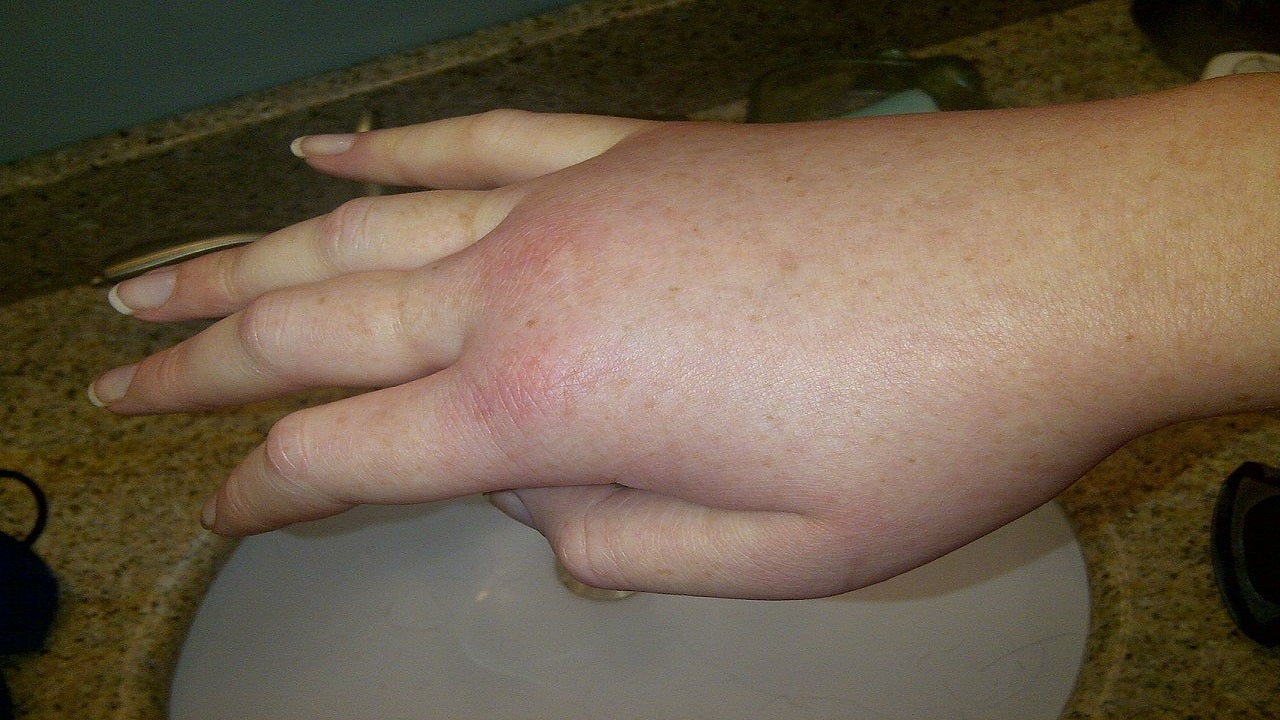
Hereditary angioedema causes and symptoms
HAE is a rare, genetic and potentially life-threatening condition caused by the deficiency of a C1 esterase inhibitor (C1-INH) enzyme. C1-INH inhibits plasma kallikrein whose activation due to reduced levels of C1-INH is responsible for the angioedema attacks.
HAE affects one in 10,000 to one in 50,000 individuals worldwide. Patients with HAE often experience several attacks every month when left untreated, with the swelling from each attack lasting for at least three days.
The current standard of treatment for patients with HAE includes repeated infusions or injections of plasma-derived C1-INH to avoid swelling attacks, as well as injection therapy to control attacks when they occur.
Common symptoms include angioedema, abdominal pain, ascites, facial oedema, and intestinal oedema.
Patients with HAE often experience several attacks a month when left untreated, and the swelling from each attack lasts for at least three days.
Berotralstat’s mechanism of action
Berotralstat binds to plasma kallikrein and inhibits its proteolytic activity. Plasma kallikrein is a protease that cleaves high-molecular-weight-kininogen (HMWK) to produce cleaved HMWK (cHMWK) and bradykinin, a potent vasodilator that enhances vascular permeability, causing HAE-associated swelling and pain.
In patients with HAE, C1-inhibitor (C1-INH) deficiency or dysfunction can result in unregulated production of plasma kallikrein naturally, which contributes to an unregulated increase in plasma kallikrein activity, leading to angioedema attacks.
Berotralstat reduces plasma kallikrein production to regulate excess bradykinin generation in HAE patients.
Clinical trials on ORLADEYO
The FDA approval of ORLADEYO was based on results from APeX-2, a pivotal Phase III, randomised, multi-centre, placebo-controlled, double-blind, parallel-group clinical study.
The study involved 120 patients who suffered from at least two confirmed attacks during the first eight weeks of the run-in duration and received at least one dose of study drug.
Patients were randomised in 1:1:1 ratio to receive berotralstat 110mg, 150mg or placebo orally once daily with food during the 24-week treatment duration.
Other prophylactic HAE medicines used by the patients were discontinued before entering the study, although the use of rescue drugs for the treatment of severe HAE attacks was allowed.
The trial’s primary endpoint was the reduction in the HAE attack rate in the intent-to-treat (ITT) population.
ORLADEYO 150mg and 110mg dosages achieved statistically significant reductions in the frequency of HAE attacks versus placebo for the ITT population.
Reductions in attack rates were observed in the first month of treatment with ORLADEYO 150mg and 110mg and were sustained over 48 weeks. The percentage reduction in the HAE attack rate was higher with ORLADEYO (150mg and 110mg) compared to placebo, regardless of the attack rate during the run-in period.
Approximately 58% of patients who received 150mg of ORLADEYO and 51% of patients who received 110mg of ORLADEYO showed a reduction in HAE attacks equal to or more than 50% compared with the baseline.
The rate of mild to severe attacks decreased by 40% and 10% in patients receiving 150mg ORLADEYO and 110mg ORLADEYO respectively compared with placebo.
In the long-term, open-label trial APeX-S, patients who completed 48 weeks of therapy with 150mg of ORLADEYO had a mean attack rate of 0.8 attacks a month. ORLADEYO was safe and well-tolerated in both trials.
BioCryst received Orphan Drug and Sakigake designations for ORLADEYO in Japan based on data from the APeX-J and APeX-2 clinical trials.
The APeX-J trial in Japan met its primary endpoint of a reduction in HAE attacks from baseline for 150mg of ORLADEYO compared with placebo. The drug was safe and generally well-tolerated in the trial.
Common side-effects of ORLADEYO reported in patients during the trials included abdominal pain, vomiting, diarrhoea, back pain, and heartburn.