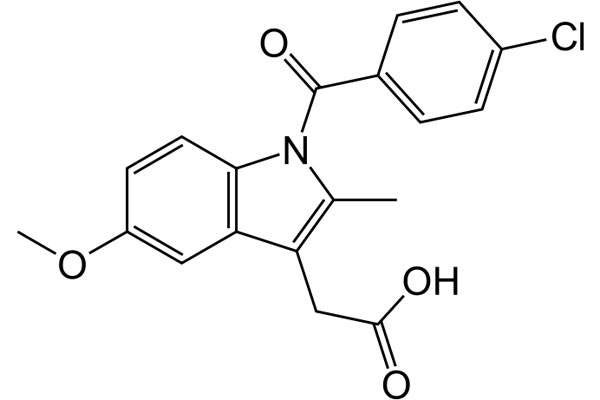
Tivorbex (indomethacin) is a nonsteroidal anti-inflammatory drug (NSAID) indicated for the treatment of mild to moderate acute pain in adults. It was discovered and developed by Iroko Pharmaceuticals.
Iroko received approval for Tivorbex from the US Food and Drug administration (FDA) for the treatment of acute pain in adults in February 2014.
Targeting acute pain: Tivorbex’s mechanism of action
Acute pain is a sudden onset of an unpleasant sensation caused by injury to body tissues. Tivorbex contains derivative non-steroidal anti-inflammatory drug (NSAID) called indometacin in the form of submicron particles which are 20 times smaller than the original size.
The drug’s precise mechanism of action is not understood completely, but it is believed to provide relief from the symptoms of inflammation and pain by inhibiting cyclooxygenase (COX-1 and COX-2) pathway.
The drug is available in capsules form for oral administration.
Clinical trials of Tivorbex
The FDA approval of Tivorbex was based on the data collected from two phase III clinical trials. Both the trials were randomised double blind and multicentre studies. The studies enrolled a total of 835 patients with post-surgical acute pain, who had a minimal pain intensity rating of at least 40mm on a 100mm visual analogue scale (VAS). The patients were administered with either Tivorbex 20mg thrice daily, 40mg twice daily, 40mg thrice daily, or placebo.
In the first clinical study, 89% of the patients in the Tivorbex 20mg thrice daily group, 90% of the patients in the Tivorbex 40mg twice daily group, 82% of the patients in the Tivorbex 40mg thrice daily group, and 97% of the patients in the placebo group used rescue medication for pain management.
In the second clinical study 87%, of the patients in the Tivorbex 20mg thrice daily group, 76% of the patients in the Tivorbex 40mg twice daily group, 80% of the patients in the Tivorbex 40mg thrice daily group, and 89% of the patients in the placebo group used rescue medication for pain management.
Results of both the clinical studies demonstrated that the patients who were administered with Tivorbex 20mg three times daily, 40mg twice-daily and 40mg three times daily demonstrated efficacy in pain intensity reduction compared to placebo. The pain intensity difference was measured over 0 to 48 hours after the first dose.
The most common adverse reactions found in the patients who used Tivorbex during the clinical trials included nausea, post procedural oedema, headache, dizziness and vomiting. The adverse reactions also included post procedural haemorrhage, constipation, diarrhoea, dyspepsia, post procedural swelling, rash, upper abdominal pain, somnolence, decreased appetite, hot flush and syncope.
Marketing commentary
Iroko Pharmaceuticals is a specialty pharmaceutical company headquartered at Navy Yard in Philadelphia. The company develops and commercialises innovative new drug products based on existing NSAIDs for pain management. The company’s sales and distribution networks for NSAIDs are spread across 40 countries.
Tivorbex is the second NSAID of Iroko Pharmaceuticals that received FDA approval, after Zorvolex in October 2013. Tivorbex is also prepared using similar proprietary SoluMatrix fine particle technology used for Zorvolex manufacturing, which was developed by Iroko Pharmaceuticals’ partner iCeutica.