Triumeq (dolutegravir/abacavir/lamivudine) is the first once-daily dolutegravir-based regimen indicated for the treatment of adult and adolescent patients (minimum age of 12 years and with a minimum weight of 40kg) affected with human immunodeficiency virus (HIV). The drug is developed and manufactured by ViiV Healthcare, a company headquartered in the UK.
Triumeq received marketing authorisation from European Commission (EC) for the treatment of HIV in adults and adolescents aged 12 years and older, and weighing at least 40kg, in September 2014.
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) provided a positive opinion for Triumeq, recommending marketing authorisation of the drug for the treatment of HIV in June 2014.
Triumeq was approved by US Food and Drug Administration (FDA) in August 2014. ViiV Healthcare submitted a new drug application (NDA) for the approval of Triumeq to the FDA in October 2013. Triumeq is also approved in Canada and is being reviewed for approval by regulatory authorities in Australia and Brazil.
Human immunodeficiency virus
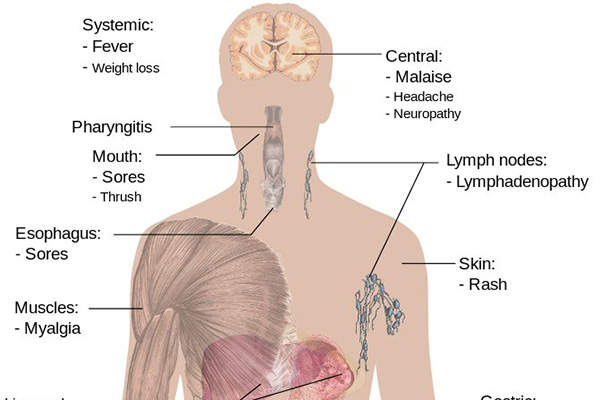
The HIV infection is caused by a virus which affects the immune system and leads to severe lifelong illness due to its long incubation period. The final stage of HIV is acquired immunodeficiency syndrome (AIDS), a condition in which the immune system is completely destroyed.
HIV is one of the most fatal communicable diseases in Europe and is often associated with a significant number of deaths and decreased life expectancy. According to the European Centre for Disease Prevention and Control, 28,038 HIV cases were reported in 29 EU/EEA member states in 2011.
Triumeq’s mechanism of action
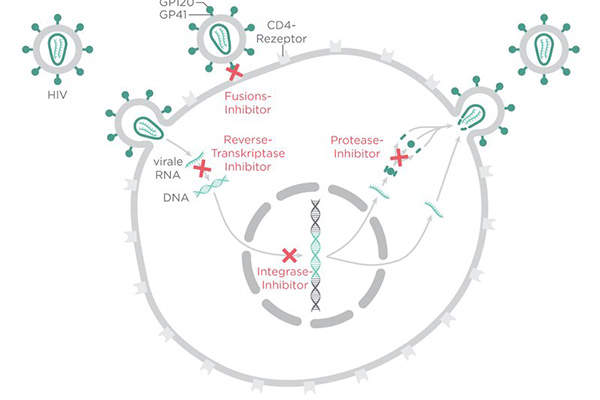
Triumeq consists of three active substances: dolutegravir, abacavir and lamivudine. Dolutegravir binds to the integrase active site and blocks the strand transfer step of retroviral DNA integration in order to inhibit HIV integrase.
Abacavir and lamivudine inhibit HIV reverse transcriptase and, by incorporating monophosphate, form a chain termination on the DNA viral chain.
Triumeq is available in the form of 50mg, 300mg and 600mg tablets for oral administration.
Clinical trials of Triumeq
ViiV Healthcare conducted Phase I clinical trials on Triumeq between June 2012 and August 2012. The open-label, single-dose, randomised, crossover study enrolled 66 healthy subjects and evaluated the bioequivalence (BE) of 50mg/600mg/300mg dolutegravir/abacavir/lamivudine tablets in comparison to one dolutegravir 50mg tablet and one EPZICOM (600mg/300mg abacavir/lamivudine) tablet.
Related content
Stribild – A Single Tablet for the Treatment of HIV-1, USA
Stribild is a combination of four compounds; elvitegravir, cobicistat, emtricitabine and tenofovir disoproxil fumarate. The drug is indicated for the treatment of HIV-1 infection in adults who have never received antiretroviral treatment for the disease.
The primary outcome measure of the study was plasma and pharmacokinetic parameters of dolutegravir/abacavir/lamivudine. Secondary outcome measures included safety and tolerability parameters as assessed by change from baseline in 12-lead ECG and vital signs.
FDA and EU approvals for Triumeq were based on data obtained from two pivotal Phase III clinical studies. The first Phase III study, called SINGLE, was conducted with dolutegravir and abacavir/lamivudine as separate pills.
The second Phase III study was a BE demonstration study, which compared the fixed-dose combination (FDC) of dolutegravir/abacavir/lamivudine, when taken as a single pill with dolutegravir and abacavir/lamivudine taken as separate pills.
The study first evaluated the BE of a FDC tablet containing 50mg of dolutegravir, 600mg of abacavir, and 300mg of lamivudine, comparing it with 50mg of dolutegravir and abacavir/lamivudine combination tablets administered at the same time. It also evaluated the effect of food on the FDC tablet.
Marketing commentary
ViiV Healthcare is a specialist HIV company established by GlaxoSmithKline and Pfizer in November 2009. The company is engaged in developing new HIV medicines and supporting people and communities living with HIV.
Other drugs approved for the treatment of HIV-1 include Sustiva (efavirenz) developed by Bristol-Myers Squibb (BMS) and DuPont Merck, Stribild manufactured by Gilead Sciences and Japan Tobacco (JT), Truvada developed by Gilead Sciences and Eviplera manufactured by Gilead Sciences International and Tibotec.