Eskata (hydrogen peroxide) for the Treatment of Raised Seborrheic Keratoses (SK), USA
Eskata® (hydrogen peroxide) is a topical, non-invasive treatment for raised seborrheic keratoses (SK) in adults.
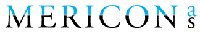
You have successfully submitted your enquiry. Someone from our company will respond ASAP
MERICON, established in 1990, offers assistance within research and development to clinical studies and regulatory affairs.
All of MERICON’s scientific staff are educated at Norwegian universities. We have experience in research and lecturing from universities in Norway and abroad. We have several years of experience from research and development within chemical and pharmaceutical industries. In addition, we have experience in consulting for governmental authorities.
MERICON offers services to the pharmaceutical industry, governmental authorities and universities.
Within pharmaceutical research and development, we are specialised in registration and clinical testing of new medicinal products with respect to efficacy and safety. We prepare registration documentation and planning, monitoring and reporting of clinical studies.
For governmental health and environmental authorities we give assistance with evaluation and reviewing of documentation.
We lecture at universities and give courses and seminars within our disciplines.
MERICON has many years’ experience in assisting pharmaceutical industry in regulatory affairs management. We can offer assistance at all levels of regulatory affairs management.
Our regulatory affairs services include:
MERICON has experience within quality assurance in connection with manufacturing of pharmaceutical ingredients according to GMP.
The clinical product development process has become a complex orchestration of individual researchers, commercial sponsors, technology providers, regulatory agencies and patient consumers. Successful outcomes are largely dependent on how people and organisations work together.
We offer a personalised service from a small team, adaptable to client requirements and with a focus on quality and scientific standards meeting the international and national requirements.
Our clinical research services include:
We have a solid foundation of knowledge in medical science and statistics, as well as extensive practical experience in clinical studies. Our statistical services include:

Eskata® (hydrogen peroxide) is a topical, non-invasive treatment for raised seborrheic keratoses (SK) in adults.
Lokelma is an oral suspension of sodium zirconium cyclosilicate that is indicated for the treatment of patients with hyperkalaemia.
Symdeko™ (tezacaftor/ivacaftor and ivacaftor) is a combination drug indicated for the treatment of cystic fibrosis (CF) in people aged 12 years and above.
Faslodex (fulvestrant) is an oestrogen receptor antagonist indicated for the treatment of locally advanced or metastatic breast cancer.
Bavencio® (avelumab) is a human PD-L1 monoclonal antibody indicated for the treatment of metastatic Merkel-cell carcinoma (MCC).

Duzallo (allopurinol and lesinurad) is indicated for the treatment of hyperuricemia associated with uncontrolled gout. It contains urate transporter-1 (URAT-1) inhibitor blended with xanthine oxidase inhibitor (XOI).
ruxima™ (rituximab) is the first biosimilar monoclonal antibody (mAb) approved for the treatment of haematological cancers, including diffuse large B-cell lymphoma, follicular lymphoma, chronic lymphocytic leukaemia, and rheumatoid arthritis, as well as granulomatosis with polyangiitis and microscopic polyangiitis.
Radicava™ (edaravone) is a neuroprotective agent indicated for the treatment of amyotrophic lateral sclerosis (ALS).
Alecensa (alectinib) is a kinase inhibitor intended for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC).
Exondys 51 (eteplirsen) is an injectable solution developed by Sarepta Therapeutics.